Levoglucosenone and Its Pseudoenantiomer iso-Levoglucosenone as Scaffolds for Drug Discovery and Development
- PMID: 32566859
- PMCID: PMC7301580
- DOI: 10.1021/acsomega.0c01331
Levoglucosenone and Its Pseudoenantiomer iso-Levoglucosenone as Scaffolds for Drug Discovery and Development
Abstract
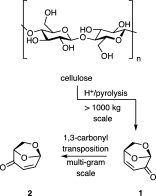
The bioderived platform molecule levoglucosenone (LGO, 1) and its readily prepared pseudoenantiomer (iso-LGO, 2) have each been subjected to α-iodination reactions with the product halides then being engaged in palladium-catalyzed Ullmann cross-coupling reactions with various bromonitropyridines. The corresponding α-pyridinylated derivatives such as 11 and 24, respectively, are produced as a result. Biological screening of such products reveals that certain of them display potent and selective antimicrobial and/or cytotoxic properties. In contrast, the azaindoles obtained by reductive cyclization of compounds such as 11 and 12 are essentially inactive in these respects. Preliminary mode-of-action studies are reported.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
-
For useful points of entry into the literature on the preparation and medicinal chemistry of azaindoles, see
- Song J. J.; Reeves J. T.; Gallou F.; Tan Z.; Yee N. K.; Senanayake C. H. Organometallic methods for the synthesis and functionalization of azaindoles. Chem. Soc. Rev. 2007, 36, 1120–1132. 10.1039/b607868k. - DOI - PubMed
- Mérour J.-Y.; Routier S.; Suzenet F.; Joseph B. Recent advances in the synthesis and properties of 4-, 5-, 6- or 7-azaindoles. Tetrahedron 2013, 69, 4767–4834. 10.1016/j.tet.2013.03.081. - DOI
- Pires M. J. D.; Poeira D. L.; Purificaçao S. I.; Marques M. M. B. Synthesis of Substituted 4-, 5-, 6- and 7-Azaindoles from Aminopyridines via a Cascade C-N Cross-Coupling/Heck Reaction. Org. Lett. 2016, 18, 3250–3253. 10.1021/acs.orglett.6b01500. - DOI - PubMed
- Philips A.; Cunningham C.; Naran K.; Kesharwanu T. Synthesis of 3-Halo-7-azaindoles through a 5-endo-dig Electrophilic Cyclization Reaction. Synlett 2019, 30, 1246–1252. 10.1055/s-0037-1611827. - DOI
- Zard S. Z. The xanthate route to pyridines. Tetrahedron 2020, 76, 13080210.1016/j.tet.2019.130802. - DOI
-
-
- Mérour J.-V.; Buron F.; Plé K.; Bonnet P.; Routier S. The Azaindole Framework in the Design of Kinase Inhibitors. Molecules 2014, 19, 19935–19979. 10.3390/molecules191219935. - DOI - PMC - PubMed
- Daydé-Cazals B.; Singer M.; Feneyrolles C.; Bestgen B.; Gassiot F.; Spenlinhauer A.; Warnault P.; Van Hijfte N.; Bornjini N.; Chevé G.; Yasri A. Rational Design, Synthesis, and Biological Evaluation of 7-Azaindole Derivatives as Potent Focused Multi-Targeted Kinase Inhibitors. J. Med. Chem. 2016, 59, 3886–3905. 10.1021/acs.jmedchem.6b00087. - DOI - PubMed
- Irie T.; Sawa M. 7-Azaindole: A Versatile Scaffold for Developing Kinase Inhibitors. Chem. Pharm Bull. 2018, 66, 29–36. 10.1248/cpb.c17-00380. - DOI - PubMed
- Machiraju P. K.; Yedla P.; Gubbala S. P.; Bohari T.; Abdul J. K. V.; Shili X.; Patel R.; Chittireddy V. R. R.; Boppana K.; Jagarlapudi S. A. R. P.; Neamatic N.; Syed R.; Amanchy R. Identification, synthesis and evaluation of CSF1R inhibitors using fragment based drug design. Comput. Biol. Chem. 2019, 80, 374–383. 10.1016/j.compbiolchem.2019.04.015. - DOI - PubMed
-
- Clark M.; Ledeboer M.; Davies I.; Byrn R. A.; Jones S. M.; Perola E.; Tsai A.; Jacobs M.; Nti-Addae K.; Bandarage U. K.; Boyd M. J.; Bethiel R. S.; Court J. J.; Deng H.; Duffy J. P.; Dorsch W. A.; Farmer L. J.; Gao H.; Gu W.; Jackson K.; Jacobs D. H.; Kennedy J. M.; Ledford B.; Liang J.; Maltais F.; Murcko M.; Wang T.; Wannamaker M. W.; Bennett H. B.; Leeman J. R.; McNeil C.; Taylor W. P.; Memmott C.; Jiang M.; Rijnbrand R.; Bral C.; Germann U.; Nezami A.; Zhang Y.; Salituro F. G.; Bennani Y. L.; Charifson P. Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J. Med. Chem. 2014, 57, 6668–6678. 10.1021/jm5007275. - DOI - PubMed
- Bandarage U. P.; Clark M. P.; Perola E.; Gao H.; Jacobs M. D.; Tsai A.; Gillespie J.; Kennedy J. M.; Maltais F.; Lededoer M. W.; Davies I.; Gu W.; Byrn R. A.; Addae K. N.; Bennett H.; Leeman J. R.; Jones S. M.; O’Brien C.; Memmott C.; Bennani Y.; Charifson P. S. Novel 2-Substituted 7-Azaindole and 7-Azaindazole Analogues as Potential Antiviral Agents for the Treatment of Influenza. ACS Med. Chem. Lett. 2017, 8, 261–265. 10.1021/acsmedchemlett.6b00487. - DOI - PMC - PubMed
- Verdonck S.; Pu S.-Y.; Sorrell F. J.; Elkins J. M.; Froeyen M.; Gao L.-J.; Prugar L. I.; Dorosky D. E.; Brannan J. M.; Barouch-Bentov R.; Knall S.; Dye J. M.; Herdewijn P.; Einav S.; De Jonghe S. Synthesis and Structure-Activity Relationships of 3,5-Disubstituted-pyrrolo[2,3-b]pyridines as Inhibitors of Adaptor-Associated Kinase 1 with Antiviral Activity. J. Med. Chem. 2019, 62, 5810–5831. 10.1021/acs.jmedchem.9b00136. - DOI - PMC - PubMed
-
- Goundry W. R. F.; Dai K.; Gonzalez M.; Legg D.; O’Kearney-McMullan A.; Morrison J.; Stark A.; Siedlecki P.; Tomlin P.; Yang J. Development and Scale-up of a Route to ATR Inhibitor AZD6738. Org. Process Res. Dev. 2019, 23, 1333–1342. 10.1021/acs.oprd.9b00075. - DOI
-
- Gluszok S.; Goossens L.; Depreux P.; Barbry D.; Hénichart J.-P. Synthesis of the 7-Azaindole (1H-Pyrrolo[2,3-b]pyridine) Analogous to Cannabimimetic JHW 200. Synth. Commun. 2006, 36, 2797–2805. 10.1080/00397910600767504. - DOI
- Immadi S. S.; Dopart R.; We Z.; Fu B.; Kendall D. A.; Lu D. Exploring 6-Azaindole and 7-Azaindole Rings for Developing Cannabinoid Receptor 1 Allosteric Modulators. Cannabis Cannabinoid Res. 2018, 3, 252–258. 10.1089/can.2018.0046. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

