Perspectives on the development of neutralizing antibodies against SARS-CoV-2
- PMID: 32566896
- PMCID: PMC7291920
- DOI: 10.1093/abt/tbaa009
Perspectives on the development of neutralizing antibodies against SARS-CoV-2
Abstract
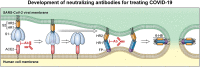
SARS-CoV-2 gains entry to human cells through its spike (S) protein binding to angiotensin-converting enzyme 2 (ACE2). Therefore, the receptor binding domain (RBD) of the S protein is the primary target for neutralizing antibodies. Selection of broad-neutralizing antibodies against SARS-CoV-2 and SARS-CoV is attractive and might be useful for treating not only COVID-19 but also future SARS-related CoV infections. Broad-neutralizing antibodies, such as 47D11, S309, and VHH-72, have been reported to target a conserved region in the RBD of the S1 subunit. The S2 subunit required for viral membrane fusion might be another target. Due to their small size and high stability, single-domain antibodies might have the ability to be administered by an inhaler making them potentially attractive therapeutics for respiratory infections. A cocktail strategy combining two (or more) antibodies that recognize different parts of the viral surface that interact with human cells might be the most effective.
Keywords: COVID-19; SARS-CoV; SARS-CoV-2; neutralizing antibody; spike (S) protein.
Published by Oxford University Press 2020.
Figures
References
-
- Cohen, J. The race is on for antibodies that stop the new coronavirus. Science 2020; 368: 564–5. - PubMed
-
- Ku, Z, Ye, X, Toa Salazar, G, et al. Antibody therapies for the treatment of COVID-19. Antib Ther 2020; 3: 101–8.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
