Physiologically Based Population Pharmacokinetic Modeling Approach for Ciprofloxacin in Bone of Patients Undergoing Orthopedic Surgery
- PMID: 32566910
- PMCID: PMC7296545
- DOI: 10.1021/acsptsci.0c00045
Physiologically Based Population Pharmacokinetic Modeling Approach for Ciprofloxacin in Bone of Patients Undergoing Orthopedic Surgery
Abstract
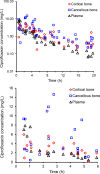
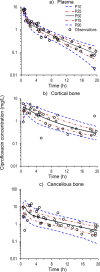
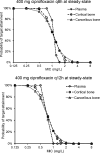
Ciprofloxacin is highly active against bacteria that commonly cause bone infections. However, the time-course of ciprofloxacin in bone has not been characterized using population pharmacokinetic modeling. Thirty-nine patients received a 1-h infusion of 400 mg of ciprofloxacin before orthopedic surgery. Blood and bone samples were collected at 0.5 to 20 h following the start of the infusion. Bone samples were separated into cortical and cancellous bone and pulverized under liquid nitrogen using a cryogenic mill. Ciprofloxacin in plasma, and cortical and cancellous bone was quantified by liquid chromatography-tandem mass spectrometry. A physiologically based pharmacokinetic modeling approach was utilized to describe the concentration-time profiles in plasma and bone. Ciprofloxacin concentrations ranged from 0.176 to 5.98 mg/L (median, 1.67; density, 1.99 g/cm3) in cortical, and 0.224 to 14.6 mg/L (median, 1.22; 1.92 g/cm3) in cancellous bone. The average observed cortical bone/plasma concentration ratio was 0.67 at 0.5 to 2 h (n = 7) and 5.1 at 13 to 20 h (n = 9). For cancellous bone the respective average ratios were 0.77 and 4.4. The population PK model included a central (blood) compartment, two peripheral tissue compartments, and compartments for the organic and inorganic (hydroxyapatite) matrix in cortical and cancellous bone. The population mean ciprofloxacin clearance was 20.7 L/h. The estimated partition coefficients of the organic bone matrix were 3.39 for cortical and 5.11 for cancellous bone. Ciprofloxacin achieved higher concentrations in bone than plasma. Slow redistribution from bone to plasma may have been due to binding to the inorganic bone matrix. The developed model presents a step toward optimized antibiotic dosing in osteomyelitis.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Ceftriaxone bone penetration in patients with septic non-union of the tibia.Int J Infect Dis. 2011 Jun;15(6):e415-21. doi: 10.1016/j.ijid.2011.03.003. Epub 2011 Apr 15. Int J Infect Dis. 2011. PMID: 21497532
-
Penetration of Vancomycin into Noninfected Bone in Patients Undergoing Total Joint Arthroplasty Evaluated by a Minimal Physiologically Based Population Pharmacokinetic Modeling Approach.Mol Pharm. 2023 Mar 6;20(3):1509-1518. doi: 10.1021/acs.molpharmaceut.2c00724. Epub 2022 Dec 13. Mol Pharm. 2023. PMID: 36512679
-
Effect of the anticoagulant ethylenediamine tetra-acetic acid (EDTA) on the estimation of pharmacokinetic parameters: A case study with tigecycline and ciprofloxacin.Xenobiotica. 2008 Jan;38(1):76-86. doi: 10.1080/00498250701678955. Xenobiotica. 2008. PMID: 17963190
-
[Concentration of ciprofloxacin in bone tissue].Aktuelle Traumatol. 1993 Apr;23(2):80-4. Aktuelle Traumatol. 1993. PMID: 8098576 German.
-
A Physiologically-Based Pharmacokinetic Model to Describe Ciprofloxacin Pharmacokinetics Over the Entire Span of Life.Clin Pharmacokinet. 2018 Dec;57(12):1613-1634. doi: 10.1007/s40262-018-0661-6. Clin Pharmacokinet. 2018. PMID: 29737457 Free PMC article. Review.
Cited by
-
Bridging the Gap Between hiPSC-CMs Cardiotoxicity Assessment and Clinical LVEF Decline Risk: A Case Study of 21 Tyrosine Kinase Inhibitors.Pharmaceuticals (Basel). 2025 Mar 23;18(4):450. doi: 10.3390/ph18040450. Pharmaceuticals (Basel). 2025. PMID: 40283889 Free PMC article.
-
Exploiting phage-antibiotic synergies to disrupt Pseudomonas aeruginosa PAO1 biofilms in the context of orthopedic infections.Microbiol Spectr. 2024 Jan 11;12(1):e0321923. doi: 10.1128/spectrum.03219-23. Epub 2023 Dec 12. Microbiol Spectr. 2024. PMID: 38084971 Free PMC article.
-
Model-Informed Precision Dosing of Antibiotics in Osteoarticular Infections.Infect Drug Resist. 2022 Jan 11;15:99-110. doi: 10.2147/IDR.S332366. eCollection 2022. Infect Drug Resist. 2022. PMID: 35046675 Free PMC article. Review.
-
Development of Hybrid Implantable Local Release Systems Based on PLGA Nanoparticles with Applications in Bone Diseases.Polymers (Basel). 2024 Oct 31;16(21):3064. doi: 10.3390/polym16213064. Polymers (Basel). 2024. PMID: 39518273 Free PMC article.
-
Spatial quantitation of antibiotics in bone tissue compartments by laser-capture microdissection coupled with UHPLC-tandem mass spectrometry.Anal Bioanal Chem. 2022 Sep;414(23):6919-6927. doi: 10.1007/s00216-022-04257-3. Epub 2022 Aug 10. Anal Bioanal Chem. 2022. PMID: 35945288 Free PMC article.
References
-
- Masters E. A.; Trombetta R. P.; de Mesy Bentley K. L.; Boyce B. F.; Gill A. L.; Gill S. R.; Nishitani K.; Ishikawa M.; Morita Y.; Ito H.; Bello-Irizarry S. N.; Ninomiya M.; Brodell J. D. Jr.; Lee C. C.; Hao S. P.; Oh I.; Xie C.; Awad H. A.; Daiss J. L.; Owen J. R.; Kates S. L.; Schwarz E. M.; Muthukrishnan G. (2019) Evolving concepts in bone infection: redefining ″biofilm″, ″acute vs. chronic osteomyelitis″, ″the immune proteome″ and ″local antibiotic therapy″. Bone Res. 7, 20.10.1038/s41413-019-0061-z. - DOI - PMC - PubMed
-
- Kavanagh N.; Ryan E. J.; Widaa A.; Sexton G.; Fennell J.; O’Rourke S.; Cahill K. C.; Kearney C. J.; O’Brien F. J.; Kerrigan S. W. (2018) Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions. Clin. Microbiol. Rev. 31, e00084–17. 10.1128/CMR.00084-17. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
