Molecular Pathogenesis and Interventional Strategies for Alzheimer's Disease: Promises and Pitfalls
- PMID: 32566913
- PMCID: PMC7296537
- DOI: 10.1021/acsptsci.9b00104
Molecular Pathogenesis and Interventional Strategies for Alzheimer's Disease: Promises and Pitfalls
Abstract
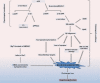
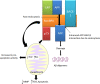
Alzheimer's disease (AD) is a debilitating disorder characterized by age-related dementia, which has no effective treatment to date. β-Amyloid depositions and hyperphosphorylated tau proteins are the main pathological hallmarks, along with oxidative stress, N-methyl-d-aspartate (NMDA) receptor-mediated excitotoxicity, and low levels of acetylcholine. Current pharmacotherapy for AD only provides symptomatic relief and limited improvement in cognitive functions. Many molecules have been explored that show promising outcomes in AD therapy and can regulate cellular survival through different pathways. To have a vivid approach to strategize the treatment regimen, AD physiopathology should be better explained considering diverse etiological factors in conjunction with biochemical disturbances. This Review attempts to discuss different disease modification approaches and address the novel therapeutic targets of AD that might pave the way for new drug discovery using the well-defined targets for therapy of the disease.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Begum M.; Biswas K.; Sarker A.; Huq T.; Sarwar A.; et al. (2015) Anticholinesterase and Antioxidant Potentials of a Medicinal Plant Abroma augusta: Implications for the Alternative Treatment Therapy of Cognitive Deficits in Alzheimer’s disease. Clin. Pharmacol. Biopharm. 4, 2.10.4172/2167-065X.1000148. - DOI
-
- Prince M., Wilmo A., Guerchet M., Ali G.-C., Wu Y.-T., and Prina M. (2015) World Alzheimer Report 2015, The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends, https://www.alz.co.uk/research/world-report-2015.
-
- El-Hayek Y. H.; Wiley R. E.; Khoury C. P.; Daya R. P.; Ballard C.; Evans A. R.; Karran M.; Molinuevo J. L.; Norton M.; Atri A. (2019) Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J. Alzheimer’s Dis. 70, 323.10.3233/JAD-190426. - DOI - PMC - PubMed
-
- The BootsWebMD, In Alzheimer's Disease guide, (2009), U.K.
Publication types
LinkOut - more resources
Full Text Sources
