Experimental and Computational Investigations of the Reactions between α,β-Unsaturated Lactones and 1,3-Dienes by Cooperative Lewis Acid/Brønsted Acid Catalysis
- PMID: 32567075
- PMCID: PMC7589441
- DOI: 10.1002/anie.202008365
Experimental and Computational Investigations of the Reactions between α,β-Unsaturated Lactones and 1,3-Dienes by Cooperative Lewis Acid/Brønsted Acid Catalysis
Abstract
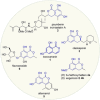
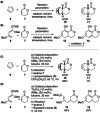
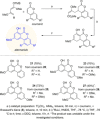
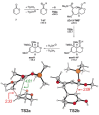
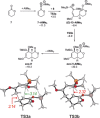
The reactions of α,β-unsaturated δ-lactones with activated dienes such as 1,3-dimethoxy-1-[(trimethylsilyl)oxy]-1,3-butadiene (Brassard's diene) are barely known in literature and show high potential for the synthesis of isocoumarin moieties. An in-depth investigation of this reaction proved a stepwise mechanism via the vinylogous Michael-products. Subsequent cyclisation and oxidation by LHMDS and DDQ, respectively, provided six mellein derivatives (30-84 %) and four angelicoin derivatives (40-78 %) over three steps. DFT-calculations provide insights into the reaction mechanism and support the theory of a stepwise reaction.
Keywords: Michael additions; catalysis; computational chemistry; density functional calculations; natural products.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

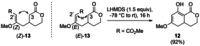





Similar articles
-
Theoretical investigations on the mechanism of hetero-Diels-Alder reactions of Brassard's diene and 1, 3-butadiene catalyzed by a tridentate schiff base titanium(IV) complex.Chemistry. 2010 Apr 12;16(14):4359-67. doi: 10.1002/chem.200902488. Chemistry. 2010. PMID: 20209517
-
Mild and highly enantioselective vinylogous aldol reaction of Brassard's diene with aromatic aldehydes by combined Lewis acid catalyst.J Org Chem. 2010 Aug 6;75(15):5326-9. doi: 10.1021/jo100674f. J Org Chem. 2010. PMID: 20590085
-
Enantioselective catalysis of the hetero-Diels-Alder reaction between Brassard's diene and aldehydes by hydrogen-bonding activation: a one-step synthesis of (S)-(+)-dihydrokawain.Chemistry. 2004 Nov 19;10(23):5964-70. doi: 10.1002/chem.200400515. Chemistry. 2004. PMID: 15487027
-
Asymmetric Catalytic Formal 1,4-Allylation of β,γ-Unsaturated α-Ketoesters: Allylboration/Oxy-Cope Rearrangement.Angew Chem Int Ed Engl. 2019 Aug 19;58(34):11846-11851. doi: 10.1002/anie.201905533. Epub 2019 Jul 12. Angew Chem Int Ed Engl. 2019. PMID: 31237402 Review.
-
Mannich-Michael versus formal aza-Diels-Alder approaches to piperidine derivatives.Org Biomol Chem. 2011 May 7;9(9):3105-21. doi: 10.1039/c0ob00996b. Epub 2011 Mar 9. Org Biomol Chem. 2011. PMID: 21390382 Review.
Cited by
-
Isocoumarins: a new class of selective carbonic anhydrase IX and XII inhibitors.J Enzyme Inhib Med Chem. 2022 Dec;37(1):743-748. doi: 10.1080/14756366.2022.2041630. J Enzyme Inhib Med Chem. 2022. PMID: 35188025 Free PMC article.
-
Natural resorcylic lactones derived from alternariol.Beilstein J Org Chem. 2024 Aug 30;20:2171-2207. doi: 10.3762/bjoc.20.187. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39224229 Free PMC article. Review.
References
-
- Huang X., Shao N., Huryk R., Palani A., Aslanian R., Seidel-Dugan C., Org. Lett. 2009, 11, 867–870. - PubMed
-
- Jiang X., García-Fortanet J., De Brabander J. K., J. Am. Chem. Soc. 2005, 127, 11254–11255. - PubMed
-
- Wan S., Wu F., Rech J. C., Green M. E., Balachandran R., Horne W. S., Day B. W., Floreancig P. E., J. Am. Chem. Soc. 2011, 133, 16668–16679. - PubMed
-
- Yu J., Yang M., Guo Y., Ye T., Org. Lett. 2019, 21, 3670–3673. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

