Overproduced bone marrow neutrophils in collagen-induced arthritis are primed for NETosis: An ignored pathological cell involving inflammatory arthritis
- PMID: 32567730
- PMCID: PMC7377937
- DOI: 10.1111/cpr.12824
Overproduced bone marrow neutrophils in collagen-induced arthritis are primed for NETosis: An ignored pathological cell involving inflammatory arthritis
Abstract
Objectives: Bone marrow edema is a universal manifestation of rheumatoid arthritis (RA), and its pathological essence is a bone marrow lesion (BML) formed by various bone marrow (BM) immune cells. Neutrophils play an important role in inflammatory arthritis, but the role and mechanism of neutrophils in BML are not clear.
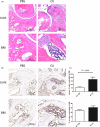
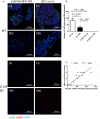
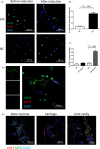
Materials and methods: Granulocyte colony-stimulating factor (G-CSF) -/- mice and wild type (WT) C57BL/6 mice were immunized for collagen-induced arthritis (CIA). Histological scores of arthritis were evaluated. Immunohistochemistry staining with anti-Ly6G was conducted. Neutrophil extracellular traps (NETs) in joint sections were determined by immunofluorescence staining. BM neutrophils were isolated for flow cytometry and NETosis induction in vitro.
Results: Histological study showed significant neutrophil infiltrations in BML of CIA mice. Inhibition of BM neutrophil production by G-CSF knock out can obstruct the induction of BML and CIA. In addition to abundant infiltrated NETs intra-articular, remarkable NETosis primed BM neutrophils were infiltrated in BML of CIA mice, which was positively related to bone erosion. Neutrophils derived from G-CSF-/- mice have diminished ability of NETs formation in vitro, while G-CSF induction can enhance its capacity of NETs formation.
Conclusions: We propose for the first time that the overproduced BM neutrophils in CIA mice are primed for NETosis in a G-CSF dependent manner, and these pathogenic cells may have an important role in inflammatory arthritis. Blocking this pathological process could be a potential strategy for the treatment of RA.
Keywords: G-CSF; bone marrow lesion; neutrophil extracellular traps; rheumatoid arthritis.
© 2020 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Inhibition of NET formation by polydatin protects against collagen-induced arthritis.Int Immunopharmacol. 2019 Dec;77:105919. doi: 10.1016/j.intimp.2019.105919. Epub 2019 Oct 23. Int Immunopharmacol. 2019. PMID: 31655341
-
Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis.Rheumatology (Oxford). 2017 Apr 1;56(4):644-653. doi: 10.1093/rheumatology/kew449. Rheumatology (Oxford). 2017. PMID: 28013195 Free PMC article.
-
Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation.Eur J Immunol. 2016 Nov;46(11):2542-2554. doi: 10.1002/eji.201646542. Epub 2016 Oct 5. Eur J Immunol. 2016. PMID: 27585946 Free PMC article.
-
Granulocyte colony-stimulating factor and neutrophils--forgotten mediators of inflammatory disease.Nat Clin Pract Rheumatol. 2006 Sep;2(9):500-10. doi: 10.1038/ncprheum0291. Nat Clin Pract Rheumatol. 2006. PMID: 16951705 Review.
-
Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review.Autoimmun Rev. 2017 Nov;16(11):1160-1173. doi: 10.1016/j.autrev.2017.09.012. Epub 2017 Sep 9. Autoimmun Rev. 2017. PMID: 28899799 Review.
Cited by
-
A lesion-selective albumin-CTLA4Ig as a safe and effective treatment for collagen-induced arthritis.Inflamm Regen. 2023 Feb 16;43(1):13. doi: 10.1186/s41232-023-00264-8. Inflamm Regen. 2023. PMID: 36797799 Free PMC article.
-
Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19.J Nanobiotechnology. 2021 Jun 10;19(1):173. doi: 10.1186/s12951-021-00926-0. J Nanobiotechnology. 2021. PMID: 34112203 Free PMC article.
-
NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps.Cancer Cell. 2023 Mar 13;41(3):505-526. doi: 10.1016/j.ccell.2023.02.001. Epub 2023 Feb 23. Cancer Cell. 2023. PMID: 36827980 Free PMC article. Review.
-
Global research trends and focus on the link between rheumatoid arthritis and neutrophil extracellular traps: a bibliometric analysis from 1985 to 2023.Front Immunol. 2023 Aug 23;14:1205445. doi: 10.3389/fimmu.2023.1205445. eCollection 2023. Front Immunol. 2023. PMID: 37680637 Free PMC article.
-
Dual Nature of Neutrophil Extracellular Traps (NETs)-From Cancer's Ally to Therapeutic Target.Cells. 2025 Aug 5;14(15):1200. doi: 10.3390/cells14151200. Cells. 2025. PMID: 40801632 Free PMC article. Review.
References
-
- Jimenez‐Boj E, Redlich K, Türk B, et al. Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J Immunol. 2005;175:2579‐2588. - PubMed
-
- Manara M, Varenna M. A clinical overview of bone marrow edema. Reumatismo. 2014;66:184‐196. - PubMed
-
- Narváez J, Sirvent E, Narváez JA, et al. Usefulness of magnetic resonance imaging of the hand versus anticyclic citrullinated peptide antibody testing to confirm the diagnosis of clinically suspected early rheumatoid arthritis in the absence of rheumatoid factor and radiographic erosions. Semin Arthritis Rheum. 2008;38:101‐109. - PubMed
-
- Lisbona MP, Pàmies A, Ares J, et al. Association of bone edema with the progression of bone erosions quantified by hand magnetic resonance imaging in patients with rheumatoid arthritis in remission. J Rheumatol. 2014;41:1623‐1629. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

