Astrocyte-derived VEGF increases cerebral microvascular permeability under high salt conditions
- PMID: 32568100
- PMCID: PMC7343440
- DOI: 10.18632/aging.103348
Astrocyte-derived VEGF increases cerebral microvascular permeability under high salt conditions
Abstract
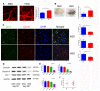
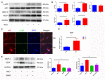
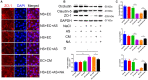
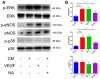
Excess salt (NaCl) intake is closely related to a variety of central nervous system (CNS) diseases characterized by increased cerebral microvascular permeability. However, the link between a high salt diet (HSD) and the breakdown of tight junctions (TJs) remains unclear. In the present study, we found that high salt does not directly influence the barrier between endothelial cells, but it suppresses expression of TJ proteins when endothelial cells are co-cultured with astrocytes. This effect is independent of blood pressure, but depends on the astrocyte activation via the NFκB/MMP-9 signaling pathway, resulting in a marked increase in VEGF expression. VEGF, in turn, induces disruption of TJs by inducing phosphorylation and activation of ERK and eNOS. Correspondingly, the HSD-induced disruption of TJ proteins is attenuated by blocking VEGF using the specific monoclonal antibody Bevacizumab. These results reveal a new axis linking a HSD to increased cerebral microvascular permeability through a VEGF-initiated inflammatory response, which may be a potential target for preventing the deleterious effects of HSD on the CNS.
Keywords: VEGF; astrocyte; cerebral microvascular permeability; high salt.
Conflict of interest statement
Figures


Similar articles
-
Apelin-13 Protects against Ischemic Blood-Brain Barrier Damage through the Effects of Aquaporin-4.Cerebrovasc Dis. 2017;44(1-2):10-25. doi: 10.1159/000460261. Epub 2017 Apr 13. Cerebrovasc Dis. 2017. PMID: 28402976
-
Mesenchymal stem cells stabilize the blood-brain barrier through regulation of astrocytes.Stem Cell Res Ther. 2015 Sep 29;6:187. doi: 10.1186/s13287-015-0180-4. Stem Cell Res Ther. 2015. PMID: 26420371 Free PMC article.
-
Gold Nanoparticles Increase Endothelial Paracellular Permeability by Altering Components of Endothelial Tight Junctions, and Increase Blood-Brain Barrier Permeability in Mice.Toxicol Sci. 2015 Nov;148(1):192-203. doi: 10.1093/toxsci/kfv176. Epub 2015 Aug 13. Toxicol Sci. 2015. PMID: 26272951
-
Genes that Mediate Metastasis across the Blood-Brain Barrier.Trends Cancer. 2020 Aug;6(8):660-676. doi: 10.1016/j.trecan.2020.04.007. Epub 2020 May 13. Trends Cancer. 2020. PMID: 32417182 Free PMC article. Review.
-
[Research progress on the effects of plateau hypoxia on blood-brain barrier structure and drug permeability].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 Dec 25;48(6):668-673. doi: 10.3785/j.issn.1008-9292.2019.12.12. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31955542 Free PMC article. Review. Chinese.
Cited by
-
Rnf-213 Knockout Induces Pericyte Reduction and Blood-Brain Barrier Impairment in Mouse.Mol Neurobiol. 2023 Nov;60(11):6188-6200. doi: 10.1007/s12035-023-03480-y. Epub 2023 Jul 12. Mol Neurobiol. 2023. PMID: 37438553
-
Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a CCL2-CCR2 pathway and NO dysregulation.Cell Rep. 2024 May 28;43(5):114193. doi: 10.1016/j.celrep.2024.114193. Epub 2024 May 5. Cell Rep. 2024. PMID: 38709635 Free PMC article.
-
Astrocyte Involvement in Blood-Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions.Int J Mol Sci. 2023 Dec 5;24(24):17146. doi: 10.3390/ijms242417146. Int J Mol Sci. 2023. PMID: 38138976 Free PMC article. Review.
-
Chronic stress-induced depression requires the recruitment of peripheral Th17 cells into the brain.J Neuroinflammation. 2022 Jul 14;19(1):186. doi: 10.1186/s12974-022-02543-6. J Neuroinflammation. 2022. PMID: 35836182 Free PMC article.
-
Research developments in the neurovascular unit and the blood‑brain barrier (Review).Biomed Rep. 2025 Mar 18;22(5):88. doi: 10.3892/br.2025.1966. eCollection 2025 May. Biomed Rep. 2025. PMID: 40166412 Free PMC article. Review.
References
-
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, et al.. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009; 15:545–52. 10.1038/nm.1960 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

