Guideline on diagnostic procedures for suspected hypersensitivity to beta-lactam antibiotics: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Society of Allergology (AeDA), German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Austrian Society for Allergology and Immunology (ÖGAI), and the Paul-Ehrlich Society for Chemotherapy (PEG)
- PMID: 32568254
- PMCID: PMC7304290
- DOI: 10.5414/ALX02104E
Guideline on diagnostic procedures for suspected hypersensitivity to beta-lactam antibiotics: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Society of Allergology (AeDA), German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Austrian Society for Allergology and Immunology (ÖGAI), and the Paul-Ehrlich Society for Chemotherapy (PEG)
Abstract
This guideline on diagnostic procedures for suspected beta-lactam antibiotic (BLA) hypersensitivity was written by the German and Austrian professional associations for allergology, and the Paul-Ehrlich Society for Chemotherapy in a consensus procedure according to the criteria of the German Association of Scientific Medical Societies. BLA such as penicillins and cephalosporins represent the drug group that most frequently triggers drug allergies. However, the frequency of reports of suspected allergy in patient histories clearly exceeds the number of confirmed cases. The large number of suspected BLA allergies has a significant impact on, e.g., the quality of treatment received by the individual patient and the costs to society as a whole. Allergies to BLA are based on different immunological mechanisms and often manifest as maculopapular exanthema, as well as anaphylaxis; and there are also a number of less frequent special clinical manifestations of drug allergic reactions. All BLA have a beta-lactam ring. BLA are categorized into different classes: penicillins, cephalosporins, carbapenems, monobactams, and beta-lactamase inhibitors with different chemical structures. Knowledge of possible cross-reactivity is of considerable clinical significance. Whereas allergy to the common beta-lactam ring occurs in only a small percentage of all BLA allergic patients, cross-reactivity due to side chain similarities, such as aminopenicillins and aminocephalosporins, and even methoxyimino cephalosporins, are more common. However, the overall picture is complex and its elucidation may require further research. Diagnostic procedures used in BLA allergy are usually made up of four components: patient history, laboratory diagnostics, skin testing (which is particularly important), and drug provocation testing. The diagnostic approach - even in cases where the need to administer a BLA is acute - is guided by patient history and risk - benefit ratio in the individual case. Here again, further studies are required to extend the present state of knowledge. Performing allergy testing for suspected BLA hypersensitivity is urgently recommended not only in the interests of providing the patient with good medical care, but also due to the immense impact of putative BLA allergies on society as a whole.
Keywords: allergy; beta-lactam antibiotics; cephalosporin; drug hypersensitivity; penicillin.
© Dustri-Verlag Dr. K. Feistle.
Figures

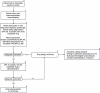
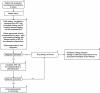



Republished from
- 10.1007/s40629-019-0100-8
References
-
- Torres Maria J Mayorga C Blanca-López N Blanca M Hypersensitivity Reactions to Beta-lactams. In: Martin SF (ed).T Lymphocytes as Tools in Diagnostics and Immunotoxicology. Basel: Springer; 2014. p. 165-184. - PubMed
-
- Gomes E Cardoso MF Praca F Gomes L Marino E Demoly P Self-reported drug allergy in general adult Portuguese population. Clin Exp Allergy. 2004; 34: 1597–1601. - PubMed
-
- Macy E Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep. 2014; 14: 476. - PubMed
-
- Rebelo Gomes E Fonseca J Araujo L Demoly P Drug allergy claims in children: from self-reporting to confirmed diagnosis. Clin Exp Allergy. 2008; 38: 191–198. - PubMed
-
- Doña I Blanca-López N Torres MJ García-Campos J García-Núñez I Gómez F Salas M Rondón C Canto MG Blanca M Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012; 22: 363–371. - PubMed
LinkOut - more resources
Full Text Sources
