A Drug Holiday Reduces the Frequency and Severity of Medication-Related Osteonecrosis of the Jaw in a Minipig Model
- PMID: 32568416
- PMCID: PMC7689727
- DOI: 10.1002/jbmr.4119
A Drug Holiday Reduces the Frequency and Severity of Medication-Related Osteonecrosis of the Jaw in a Minipig Model
Abstract
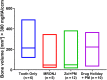
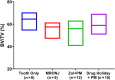
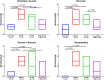
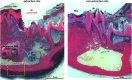
Treatment of medication-related osteonecrosis of the jaw (MRONJ) is challenging and no clear consensus has been achieved. This study investigated preventive measures recommended for tooth extractions under antiresorptive (AR) treatment and the role of discontinuation of AR therapy to avoid the onset of MRONJ in a minipig model. Thirty-six Göttingen minipigs were divided into four groups. Group 1 (negative control): tooth extractions but no zoledronate (ZOL). Group 2 (positive control): weekly ZOL infusions for 12 weeks followed by tooth extractions without wound management followed by 8 weeks of ZOL treatment. Group 3: weekly ZOL infusions for 12 weeks followed by tooth extractions; surgical wound management (resection of sharp bone edges, mucoperiosteal coverage); and continuation of ZOL infusions for 8 weeks plus antibiotic treatment. Group 4: 12 weeks of ZOL infusions followed by a drug holiday for 6 weeks. Tooth extractions with preventive wound management followed by antibiotic treatment for 8 weeks but no ZOL infusions. Jawbones were subjected to macroscopic, radiological (CT and micro-CT) and histopathological investigations. No clinical cases of MRONJ were observed in the negative group, in the positive control all animals developed MRONJ. Group 3 developed MRONJ in 83% of cases. With a drug holiday, 40% developed MRONJ in areas of tooth extraction. This is the first large animal model that reduces the occurrence of MRONJ following tooth extraction by the implementation of a drug holiday combined with antibiotic prophylaxis and smoothening of sharp bony edges. © 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research..
Keywords: ANIMAL MODEL; BISPHOSPHONATES; BRONJ; MINIPIG; MRONJ; OSTEONECROSIS; PREVENTION; PROPHYLAXIS; ZOLEDRONATE.
© 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Figures








Similar articles
-
Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model).Medicina (Kaunas). 2023 May 22;59(5):1000. doi: 10.3390/medicina59051000. Medicina (Kaunas). 2023. PMID: 37241232 Free PMC article.
-
Further development of the MRONJ minipig large animal model.J Craniomaxillofac Surg. 2017 Sep;45(9):1503-1514. doi: 10.1016/j.jcms.2017.07.002. Epub 2017 Jul 18. J Craniomaxillofac Surg. 2017. PMID: 28803745
-
Low-level laser therapy prevents medication-related osteonecrosis of the jaw-like lesions via IL-1RA-mediated primary gingival wound healing.BMC Oral Health. 2023 Jan 10;23(1):14. doi: 10.1186/s12903-022-02678-1. BMC Oral Health. 2023. PMID: 36627695 Free PMC article.
-
Rodents as an animal model for studying tooth extraction-related medication-related osteonecrosis of the jaw: assessment of outcomes.Arch Oral Biol. 2024 Mar;159:105875. doi: 10.1016/j.archoralbio.2023.105875. Epub 2023 Dec 26. Arch Oral Biol. 2024. PMID: 38160519 Free PMC article. Review.
-
Pathogenesis of medication-related osteonecrosis of the jaw: a comparative study of in vivo and in vitro trials.J Int Med Res. 2018 Oct;46(10):4277-4296. doi: 10.1177/0300060518788987. Epub 2018 Aug 9. J Int Med Res. 2018. PMID: 30091399 Free PMC article. Review.
Cited by
-
Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model).Medicina (Kaunas). 2023 May 22;59(5):1000. doi: 10.3390/medicina59051000. Medicina (Kaunas). 2023. PMID: 37241232 Free PMC article.
-
The effect of drug holiday on preventing medication-related osteonecrosis of the jaw in osteoporotic rat model.J Orthop Translat. 2023 Jan 10;39:55-62. doi: 10.1016/j.jot.2022.12.006. eCollection 2023 Mar. J Orthop Translat. 2023. PMID: 36721766 Free PMC article.
-
Medication-Related Osteonecrosis: Why the Jawbone?Dent J (Basel). 2023 Apr 23;11(5):109. doi: 10.3390/dj11050109. Dent J (Basel). 2023. PMID: 37232760 Free PMC article. Review.
-
Non-Interventional Prospective Observational Study of Platelet Rich Fibrin as a Therapy Adjunctive in Patients with Medication-Related Osteonecrosis of the Jaw.J Clin Med. 2022 Jan 28;11(3):682. doi: 10.3390/jcm11030682. J Clin Med. 2022. PMID: 35160132 Free PMC article.
-
Minimizing MRONJ after Tooth Extraction in Cancer Patients Receiving Bone-Modifying Agents.J Clin Med. 2022 Mar 25;11(7):1807. doi: 10.3390/jcm11071807. J Clin Med. 2022. PMID: 35407415 Free PMC article.
References
-
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7. - PubMed
-
- Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46(9):1515–25. - PubMed
-
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate‐associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91. - PubMed
-
- Fliefel R, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate‐related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44(5):568–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

