Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination With Carboplatin in Patients With Advanced Solid Tumors
- PMID: 32568634
- PMCID: PMC7499606
- DOI: 10.1200/JCO.19.02404
Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination With Carboplatin in Patients With Advanced Solid Tumors
Abstract
Purpose: Preclinical studies demonstrated that ATR inhibition can exploit synthetic lethality (eg, in cancer cells with impaired compensatory DNA damage responses through ATM loss) as monotherapy and combined with DNA-damaging drugs such as carboplatin.
Patients and methods: This phase I trial assessed the ATR inhibitor M6620 (VX-970) as monotherapy (once or twice weekly) and combined with carboplatin (carboplatin on day 1 and M6620 on days 2 and 9 in 21-day cycles). Primary objectives were safety, tolerability, and maximum tolerated dose; secondary objectives included pharmacokinetics and antitumor activity; exploratory objectives included pharmacodynamics in timed paired tumor biopsies.
Results: Forty patients were enrolled; 17 received M6620 monotherapy, which was safe and well tolerated. The recommended phase II dose (RP2D) for once- or twice-weekly administration was 240 mg/m2. A patient with metastatic colorectal cancer harboring molecular aberrations, including ATM loss and an ARID1A mutation, achieved RECISTv1.1 complete response and maintained this response, with a progression-free survival of 29 months at last assessment. Twenty-three patients received M6620 with carboplatin, with mechanism-based hematologic toxicities at higher doses, requiring dose delays and reductions. The RP2D for combination therapy was M6620 90 mg/m2 with carboplatin AUC5. A patient with advanced germline BRCA1 ovarian cancer achieved RECISTv1.1 partial response and Gynecologic Cancer Intergroup CA125 response despite being platinum refractory and PARP inhibitor resistant. An additional 15 patients had RECISTv1.1 stable disease as best response. Pharmacokinetics were dose proportional and exceeded preclinical efficacious levels. Pharmacodynamic studies demonstrated substantial inhibition of phosphorylation of CHK1, the downstream ATR substrate.
Conclusion: To our knowledge, this report is the first of an ATR inhibitor as monotherapy and combined with carboplatin. M6620 was well tolerated, with target engagement and preliminary antitumor responses observed.
Figures

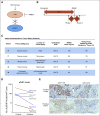
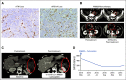

References
-
- Shiloh Y. ATM: Expanding roles as a chief guardian of genome stability. Exp Cell Res. 2014;329:154–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

