What Are the Minimum Clinically Important Differences in SF-36 Scores in Patients with Orthopaedic Oncologic Conditions?
- PMID: 32568896
- PMCID: PMC7431256
- DOI: 10.1097/CORR.0000000000001341
What Are the Minimum Clinically Important Differences in SF-36 Scores in Patients with Orthopaedic Oncologic Conditions?
Abstract
Background: The SF-36 is widely used to evaluate the health-related quality of life of patients with musculoskeletal tumors. The minimum clinically important difference (MCID) is useful for interpreting changes in functional scores because it defines the smallest change each patient may perceive. Since the MCID is influenced by the population characteristics, MCIDs of the SF-36 should be defined to reflect the specific conditions of orthopaedic oncology patients.
Questions/purposes: (1) What is the MCID of SF-36 physical component summary (PCS) and mental component summary (MCS) scores in patients with orthopaedic oncologic conditions when calculated with distribution-based methods? (2) What is the MCID of SF-36 PCS and MCS scores in patients with orthopaedic oncologic conditions when calculated by anchor-based methods?
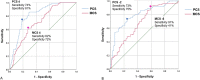
Methods: Of all 960 patients who underwent surgery from 1999 to 2005, 32% (310) of patients who underwent musculoskeletal oncologic surgery and completed two surveys during postoperative follow-up were reviewed. We evaluated a dataset that ended in 2005, completing follow-up of data accrued as part of the cooperative effort between the American Academy of Orthopaedic Surgeons and the Council of Musculoskeletal Specialty Societies to create patient reported quality of life instruments for lower extremity conditions. This effort, started in 1994 was validated and widely accepted by its publication in 2004. We believe the findings from this period are still relevant today because (1) this critical information has never been available for clinicians and researchers to distinguish real differences in outcome among orthopaedic oncology patients, (2) the SF-36 continues to be the best validated and widely used instrument to assess health-related quality of life, and unfortunately (3) there has been no significant change in outcome for oncology patients over the intervening years. SF-36 PCS and MCS are aggregates of the eight scale scores specific to physical and mental dimension (scores range from 0 to 100, with higher scores representing better health). Their responsiveness has been shown postoperatively for several surgical procedures (such as, colorectal surgery). Two different methods were used to calculate the MCID: the distribution-based method, which was based on half the SD of the change in score and standard error of the measurement at baseline, and anchor-based, in which a receiver operating characteristic (ROC) curve analysis was performed. The anchor-based method uses a plain-language question to ask patients how their individual conditions changed when compared with the previous survey. Answer choices were "much better," "somewhat better," "about the same," "somewhat worse," or "much worse." The ROC curve-derived MCIDs were defined as the change in scores from baseline, with sensitivity and specificity to detect differences in patients who stated their outcome was, about the same and those who stated their status was somewhat better or somewhat worse. This approach is based on each patient's perception. It considers that the definition of MCID is the minimal difference each patient can perceive as meaningful.
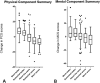
Results: Using the distribution-based method, we found that the MCIDs of the PCS and MCS were 5 and 5 by half the SD, and 6 and 5 by standard error of the measurement. In the anchor-based method, the MCIDs of the PCS and MCS for improvement/deterioration were 4 (area under the curve, 0.82)/-2 (area under the curve, 0.79) and 4 (area under the curve, 0.72)/ (area under the curve, 0.68), respectively.
Conclusions: Since both anchor-based and distribution-based MCID estimates of the SF-36 in patients with musculoskeletal tumors were so similar, we have confidence in the estimates we made, which were about 5 points for both the PCS and the MCS subscales of the SF-36. This suggests that interventions improving SF-36 by less than that amount are unlikely to be perceived by patients as clinically important. Therefore, those interventions may not justify exposing patients to risk, cost, or inconvenience. When applying new interventions to orthopaedic oncology patients going forward, it will be important to consider these MCIDs for evaluation purposes.
Level of evidence: Level III, diagnostic study.
Conflict of interest statement
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and
Figures

Comment in
-
CORR Insights®: What Are the Minimum Clinically Important Differences in SF-36 Scores in Patients with Orthopaedic Oncologic Conditions?Clin Orthop Relat Res. 2020 Sep;478(9):2159-2160. doi: 10.1097/CORR.0000000000001429. Clin Orthop Relat Res. 2020. PMID: 32769537 Free PMC article. No abstract available.
References
-
- Akiyama T, Uehara K, Ogura K, Shinoda Y, Iwata S, Saita K, Tanzawa Y, Nakatani F, Yonemoto T, Kawano H, Davis AM, Kawai A. Cross-cultural adaptation and validation of the Japanese version of the Toronto Extremity Salvage Score (TESS) for patients with malignant musculoskeletal tumors in the upper extremities. J Orthop Sci. 2017;22:127-132. - PubMed
-
- Anagnostopoulos F, Niakas D, Pappa E. Construct validation of the Greek SF-36 Health Survey. Qual Life Res. 2005;14:1959-1965. - PubMed
-
- Antonescu I, Carli F, Mayo NE, Feldman LS. Validation of the SF-36 as a measure of postoperative recovery after colorectal surgery. Surg Endosc. 2014;28:3168-3178. - PubMed
-
- Auffinger BM, Lall RR, Dahdaleh NS, Wong AP, Lam SK, Koski T, Fessler RG, Smith ZA. Measuring surgical outcomes in cervical spondylotic myelopathy patients undergoing anterior cervical discectomy and fusion: assessment of minimum clinically important difference. PLoS One. 2013;8:e67408. - PMC - PubMed
-
- Badhiwala JH, Witiw CD, Nassiri F, Akbar MA, Jaja B, Wilson JR, Fehlings MG. Minimum Clinically Important Difference in SF-36 Scores for Use in Degenerative Cervical Myelopathy. Spine (Phila Pa 1976). 2018;43:E1260-e1266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

