Studies on CRMP2 SUMOylation-deficient transgenic mice identify sex-specific Nav1.7 regulation in the pathogenesis of chronic neuropathic pain
- PMID: 32569093
- PMCID: PMC7572581
- DOI: 10.1097/j.pain.0000000000001951
Studies on CRMP2 SUMOylation-deficient transgenic mice identify sex-specific Nav1.7 regulation in the pathogenesis of chronic neuropathic pain
Abstract
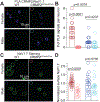
The sodium channel Nav1.7 is a master regulator of nociceptive input into the central nervous system. Mutations in this channel can result in painful conditions and produce insensitivity to pain. Despite being recognized as a "poster child" for nociceptive signaling and human pain, targeting Nav1.7 has not yet produced a clinical drug. Recent work has illuminated the Nav1.7 interactome, offering insights into the regulation of these channels and identifying potentially new druggable targets. Among the regulators of Nav1.7 is the cytosolic collapsin response mediator protein 2 (CRMP2). CRMP2, modified at lysine 374 (K374) by addition of a small ubiquitin-like modifier (SUMO), bound Nav1.7 to regulate its membrane localization and function. Corollary to this, preventing CRMP2 SUMOylation was sufficient to reverse mechanical allodynia in rats with neuropathic pain. Notably, loss of CRMP2 SUMOylation did not compromise other innate functions of CRMP2. To further elucidate the in vivo role of CRMP2 SUMOylation in pain, we generated CRMP2 K374A knock-in (CRMP2) mice in which Lys374 was replaced with Ala. CRMP2 mice had reduced Nav1.7 membrane localization and function in female, but not male, sensory neurons. Behavioral appraisal of CRMP2 mice demonstrated no changes in depressive or repetitive, compulsive-like behaviors and a decrease in noxious thermal sensitivity. No changes were observed in CRMP2 mice to inflammatory, acute, or visceral pain. By contrast, in a neuropathic model, CRMP2 mice failed to develop persistent mechanical allodynia. Our study suggests that CRMP2 SUMOylation-dependent control of peripheral Nav1.7 is a hallmark of chronic, but not physiological, neuropathic pain.
Conflict of interest statement
CONFLICT OF INTERESTS STATEMENT
R. Khanna is the co-founder of Regulonix LLC, a company developing non-opioids drugs for chronic pain. In addition, R. Khanna has patents US10287334 and US10441586 issued to Regulonix LLC. The other authors declare no competing financial interest.
Figures




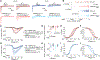


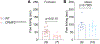


References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 2008;321(5889):702–705. - PubMed
-
- Alexandrou AJ, Brown AR, Chapman ML, Estacion M, Turner J, Mis MA, Wilbrey A, Payne EC, Gutteridge A, Cox PJ, Doyle R, Printzenhoff D, Lin Z, Marron BE, West C, Swain NA, Storer RI, Stupple PA, Castle NA, Hounshell JA, Rivara M, Randall A, Dib-Hajj SD, Krafte D, Waxman SG, Patel MK, Butt RP, Stevens EB. Subtype-Selective Small Molecule Inhibitors Reveal a Fundamental Role for Nav1.7 in Nociceptor Electrogenesis, Axonal Conduction and Presynaptic Release. PloS one 2016;11(4):e0152405. - PMC - PubMed
-
- Berta T, Poirot O, Pertin M, Ji RR, Kellenberger S, Decosterd I. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol Cell Neurosci 2008;37(2):196–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

