Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer
- PMID: 32569727
- PMCID: PMC7542577
- DOI: 10.1016/j.annonc.2020.06.003
Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer
Abstract
Background: Accumulating evidence has identified Fusobacterium as an important pathogenic gut bacterium associated with colorectal cancer. Nevertheless, only limited data exist about the role of this bacterium in locally advanced rectal cancer (LARC). In this study, we quantified Fusobacterium nucleatum in untreated and post-neoadjuvant chemoradiotherapy (nCRT) samples from LARC patients and investigated its association with therapy response and survival.
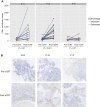
Patients and methods: A total of 254 samples from 143 patients with rectal adenocarcinomas were analyzed for the presence and abundance of F. nucleatum using RNA in situ hybridization and digital image analysis. Assay accuracy was determined using infected cell lines and tumor samples with available quantitative PCR data. We studied the impact of F. nucleatum load on pathologic complete response and relapse-free survival. Treatment-induced changes were evaluated in paired pre- and post-nCRT samples (n = 71). Finally, tumor microenvironment changes during nCRT were assessed in paired samples (n = 45) by immune contexture analysis.
Results: F. nucleatum tissue levels by RNA in situ hybridization strongly correlated with quantitative PCR (r = 0.804, P < 0.001). F. nucleatum abundance was higher in untreated [median, 7.4; 95% confidence interval (3.7-16.2)] compared with treated [median, 1.6; 95% confidence interval (1.3-2.4)] tumors (P <0.001) with 58% (73/126) and 26% (22/85) positive tumors, respectively (P < 0.001). Baseline F. nucleatum levels were not associated with pathologic complete response. F. nucleatum positivity after nCRT, but not baseline status, significantly increased risk of relapse [hazard ratio = 7.5, 95% confidence interval (3.0-19.0); P < 0.001]. Tumors that turned F. nucleatum-negative after nCRT had a strong increase in CD8+ T cells post-nCRT (P < 0.001), while those that persisted F. nucleatum-positive after nCRT lacked CD8+ T cells induction in post-nCRT samples compared with baseline (P = 0.69).
Conclusion: F. nucleatum persistence post-nCRT is associated with high relapse rates in LARC, potentially linked to suppression of immune cytotoxicity.
Keywords: Fusobacterium nucleatum; locally advanced rectal cancer; microbiome; preoperative chemoradiotherapy.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure JT reports personal financial interest in the form of scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Chugai, Genentech, Inc., Genmab A/S, Halozyme, Imugene Limited, Inflection Biosciences Limited, Ipsen, Kura Oncology, Lilly, Merck Sharp & Dohme, Menarini, Merck Serono, Merrimack, Merus, Molecular Partners, Novartis, Peptomyc, Pfizer, Pharmacyclics, ProteoDesign SL, Rafael Pharmaceuticals, F. Hoffmann-La Roche Ltd, Sanofi, SeaGen, Seattle Genetics, Servier, Symphogen, Taiho, VCN Biosciences, Biocartis, Foundation Medicine, HalioDX SAS, and Roche Diagnostics. JC reports scientific consultancy role (speaker and advisory roles) from Novartis, Pfizer, Ipsen, Exelixis, Bayer, Eisai, Advanced Accelerator Applications, Amgen, Sanofi, ITM, Sirlex and Merck Serono. Research grants from Novartis, Pfizer, AstraZeneca, Advanced Accelerator Applications, Eisai, and Bayer. RD has an advisory role at Roche and Boehringer-Ingelheim, has received a speaker’s fee from Roche, Ipsen, Amgen, Sanofi, Servier, Merck Sharp & Dohme and further received direct research funding from Merck and Pierre Fabre. PN has consulted for Bayer, Novartis, Merck Sharp & Dohme, and Targos, and received compensation. The other authors have declared no conflicts of interest.
Figures


Comment in
-
Fusobacterium nucleatum, rectal cancer and radiotherapy.Ann Oncol. 2020 Oct;31(10):1277-1278. doi: 10.1016/j.annonc.2020.06.019. Epub 2020 Jul 4. Ann Oncol. 2020. PMID: 32629022 No abstract available.
References
-
- Sauer R., Becker H., Hohenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. - PubMed
-
- Bosset J.F., Calais G., Mineur L. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results - EORTC 22921. J Clin Oncol. 2005;23(24):5620–5627. - PubMed
-
- Fokas E., Liersch T., Fietkau R. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32(15):1554–1562. - PubMed
-
- Patel U.B., Taylor F., Blomqvist L. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–3760. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

