IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA
- PMID: 32569770
- PMCID: PMC11752806
- DOI: 10.1053/j.gastro.2020.06.038
IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA
Abstract
Background & aims: Defects in the epithelial tight junction (TJ) barrier contribute to development of intestinal inflammation associated with diseases. Interleukin 1 beta (IL1B) increases intestinal permeability in mice. We investigated microRNAs that are regulated by IL1B and their effects on expression of TJ proteins and intestinal permeability.
Methods: We used Targetscan to identify microRNAs that would bind the 3' untranslated region (3'UTR) of occludin mRNA; regions that interacted with microRNAs were predicted using the V-fold server and Assemble2, and 3-dimensional models were created using UCSF Chimera linked with Assemble2. Caco-2 cells were transfected with vectors that express microRNAs, analyzed by immunoblots and real-time polymerase chain reaction (PCR), and grown as monolayers; permeability in response to IL1B was assessed with the marker inulin. Male C57BL/6 mice were given intraperitoneal injections of IL1B and intestinal recycling perfusion was measured; some mice were given dextran sodium sulfate to induce colitis and/or gavage with an antagonist to MIR200C-3p (antagomiR-200C) or the nonspecific antagomiR (control). Intestinal tissues were collected from mice and analyzed by histology and real-time PCR; enterocytes were isolated by laser capture microdissection. We also analyzed colon tissues and organoids from patients with and without ulcerative colitis.
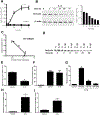
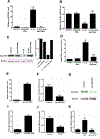
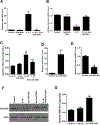
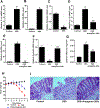
Results: Incubation of Caco-2 monolayers with IL1B increased TJ permeability and reduced levels of occludin protein and mRNA without affecting the expression of other transmembrane TJ proteins. Targetscan identified MIR122, MIR200B-3p, and MIR200C-3p, as miRNAs that might bind to the occludin 3'UTR. MIR200C-3p was rapidly increased in Caco-2 cells incubated with IL1B; the antagomiR-200c prevented the IL1B-induced decrease in occludin mRNA and protein and reduced TJ permeability. Administration of IL1B to mice increased small intestinal TJ permeability, compared with mice given vehicle; enterocytes isolated from mice given IL1B had increased expression of MIR200C-3p and decreased levels of occludin messenger RNA (mRNA) and protein. Intestinal tissues from mice with colitis had increased levels of IL1B mRNA and MIR200C-3p and decreased levels of occludin mRNA; gavage of mice with antagomiR-200C reduced levels of MIR200C-3p and prevented the decrease in occludin mRNA and the increase in colonic permeability. Colon tissues and organoids from patients with ulcerative colitis had increased levels of IL1B mRNA and MIR200C-3p compared with healthy controls. Using 3-dimensional molecular modeling and mutational analyses, we identified the nucleotide bases in the occluding mRNA 3'UTR that interact with MIR200C-3p.
Conclusions: Intestine tissues from patients with ulcerative colitis and mice with colitis have increased levels of IL1B mRNA and MIR200C-3p, which reduces expression of occludin by enterocytes and thereby increases TJ permeability. Three-dimensional modeling of the interaction between MIR200C-3p and the occludin mRNA 3'UTR identified sites of interaction. The antagomiR-200C prevents the decrease in occludin in enterocytes and intestine tissues of mice with colitis, maintaining the TJ barrier.
Keywords: Epigenetic Modification; MicroRNA 200C; RNA Degradation; Translation.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors disclose no conflicts.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

