Treatment Response of Add-On Esketamine Nasal Spray in Resistant Major Depression in Relation to Add-On Second-Generation Antipsychotic Treatment
- PMID: 32570275
- PMCID: PMC7387762
- DOI: 10.1093/ijnp/pyaa034
Treatment Response of Add-On Esketamine Nasal Spray in Resistant Major Depression in Relation to Add-On Second-Generation Antipsychotic Treatment
Abstract
In this meta-analysis, we aimed to estimate and compare the efficacy of add-on treatment of antidepressants with esketamine nasal spray and second-generation antipsychotics in patients with nonpsychotic major depressive disorder and inadequate response to antidepressants. Searching for acute-phase, double-blind, placebo-controlled, randomized trials, we found 22 second-generation antipsychotic (n = 8363) and 3 intranasal esketamine (n = 641) studies. Mean change in the Montgomery Åsberg Depression Rating Scale total score served as outcome. We determined a higher mean difference (vs placebo) for the pooled esketamine nasal spray trials (mean difference = 4.09, 95% confidence interval: 2.01 to 6.17) than for the pooled second-generation antipsychotic augmentation trials (mean difference = 2.05, 95% confidence interval: 1.51 to 2.59). Thus, the effect size for intranasal esketamine was nearly twice as high as those for the second-generation antipsychotics. This indicates high efficacy of add-on esketamine nasal spray in treatment-resistant major depressive disorder compared with other well-established, evidence-based pharmacological options such as augmentation with second-generation antipsychotics.
Keywords: add-on treatment; esketamine nasal spray; major depressive disorder; second-generation antipsychotics; treatment resistance.
© The Author(s) 2020. Published by Oxford University Press on behalf of CINP.
Figures
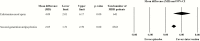

Similar articles
-
Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study.Am J Psychiatry. 2019 Jun 1;176(6):428-438. doi: 10.1176/appi.ajp.2019.19020172. Epub 2019 May 21. Am J Psychiatry. 2019. PMID: 31109201 Clinical Trial.
-
Esketamine Nasal Spray in Major Depressive Disorder: A Meta-Analysis of Randomized Controlled Trials.Clin Pharmacol Ther. 2025 Jun;117(6):1637-1649. doi: 10.1002/cpt.3555. Epub 2025 Jan 10. Clin Pharmacol Ther. 2025. PMID: 39790081 Review.
-
Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1).Int J Neuropsychopharmacol. 2019 Oct 1;22(10):616-630. doi: 10.1093/ijnp/pyz039. Int J Neuropsychopharmacol. 2019. PMID: 31290965 Free PMC article. Clinical Trial.
-
Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients With Treatment-Resistant Depression: A Randomized Clinical Trial.JAMA Psychiatry. 2019 Sep 1;76(9):893-903. doi: 10.1001/jamapsychiatry.2019.1189. JAMA Psychiatry. 2019. PMID: 31166571 Free PMC article. Clinical Trial.
-
Efficacy of esketamine nasal spray for treatment-resistant depression: A meta-analysis of randomized controlled studies.Medicine (Baltimore). 2025 Feb 28;104(9):e41495. doi: 10.1097/MD.0000000000041495. Medicine (Baltimore). 2025. PMID: 40020133 Free PMC article.
Cited by
-
Efficacy of Analgesic Propofol/Esketamine and Propofol/Fentanyl for Painless Induced Abortion: A Randomized Clinical Trial.Biomed Res Int. 2022 Jun 9;2022:5095282. doi: 10.1155/2022/5095282. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2023 Nov 29;2023:9832694. doi: 10.1155/2023/9832694. PMID: 35722469 Free PMC article. Retracted. Clinical Trial.
-
Case Report: Repeated Series of Ketamine Infusions in Patients With Treatment-Resistant Depression: Presentation of Five Cases.Front Psychiatry. 2021 Dec 2;12:705190. doi: 10.3389/fpsyt.2021.705190. eCollection 2021. Front Psychiatry. 2021. PMID: 34925081 Free PMC article.
-
Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation.Am J Psychiatry. 2021 May 1;178(5):383-399. doi: 10.1176/appi.ajp.2020.20081251. Epub 2021 Mar 17. Am J Psychiatry. 2021. PMID: 33726522 Free PMC article. Review.
-
Indirect adjusted comparison of 6-month clinical outcomes between esketamine nasal spray and other real-world polypharmacy treatment strategies for treatment resistant depression: results from the ICEBERG study.Front Psychiatry. 2023 Oct 31;14:1250987. doi: 10.3389/fpsyt.2023.1250987. eCollection 2023. Front Psychiatry. 2023. PMID: 38025416 Free PMC article.
-
Clinical Features and Outcomes of 124 Italian Patients With Treatment Resistant Depression: A Real-World, Prospective Study.Front Psychiatry. 2021 Nov 5;12:769693. doi: 10.3389/fpsyt.2021.769693. eCollection 2021. Front Psychiatry. 2021. PMID: 34803777 Free PMC article.
References
-
- Bartova L, Dold M, Kautzky A, Fabbri C, Spies M, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, Schosser A, Kasper S (2019) Results of the European Group for the Study of Resistant Depression (GSRD) - basis for further research and clinical practice. World J Biol Psychiatry 20:427–448. - PubMed
-
- Bauer M, Severus E, Möller HJ, Young AH; WFSBP Task Force on Unipolar Depressive Disorders (2017) Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract 21:166–176. - PubMed
-
- Blier P. (2014) Rational site-directed pharmacotherapy for major depressive disorder. Int J Neuropsychopharmacol 17:997–1008. - PubMed
-
- Dold M, Bartova L, Kautzky A, Serretti A, Porcelli S, Souery D, Mendlewicz J, Montgomery S, Zohar J, Kasper S (2018) Clinical factors associated with augmentation treatment with second-generation antipsychotics and lithium in major depression - results from a European multicenter study. Eur Neuropsychopharmacol 28:1305–1313. - PubMed
-
- Dold M, Kasper S (2017) Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J Psychiatry Clin Pract 21:13–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

