Rapid Direct Nucleic Acid Amplification Test without RNA Extraction for SARS-CoV-2 Using a Portable PCR Thermocycler
- PMID: 32570810
- PMCID: PMC7349311
- DOI: 10.3390/genes11060664
Rapid Direct Nucleic Acid Amplification Test without RNA Extraction for SARS-CoV-2 Using a Portable PCR Thermocycler
Abstract
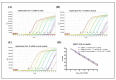
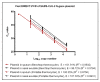
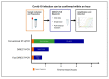
There is an ongoing worldwide coronavirus disease 2019 (Covid-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). At present, confirmatory diagnosis is by reverse transcription polymerase chain reaction (RT-PCR), typically taking several hours and requiring a molecular laboratory to perform. There is an urgent need for rapid, simplified, and cost-effective detection methods. We have developed and analytically validated a protocol for direct rapid extraction-free PCR (DIRECT-PCR) detection of SARS-CoV-2 without the need for nucleic acid purification. As few as six RNA copies per reaction of viral nucleocapsid (N) gene from respiratory samples such as sputum and nasal exudate can be detected directly using our one-step inhibitor-resistant assay. The performance of this assay was validated on a commercially available portable PCR thermocycler. Viral lysis, reverse transcription, amplification, and detection are achieved in a single-tube homogeneous reaction within 36 min. This minimizes hands-on time, reduces turnaround-time for sample-to-result, and obviates the need for RNA purification reagents. It could enable wider use of Covid-19 testing for diagnosis, screening, and research in countries and regions where laboratory capabilities are limiting.
Keywords: Covid-19; DIRECT-PCR; Diagnostics; Point-of-care; SARS-CoV-2; portable PCR.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Portable and accurate diagnostics for COVID-19: Combined use of the miniPCR thermocycler and a well-plate reader for SARS-CoV-2 virus detection.PLoS One. 2020 Aug 13;15(8):e0237418. doi: 10.1371/journal.pone.0237418. eCollection 2020. PLoS One. 2020. PMID: 32790779 Free PMC article.
-
A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2).Virology. 2020 Oct;549:1-4. doi: 10.1016/j.virol.2020.07.006. Epub 2020 Jul 29. Virology. 2020. PMID: 32758712 Free PMC article.
-
A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19.Int J Mol Sci. 2020 Jul 29;21(15):5380. doi: 10.3390/ijms21155380. Int J Mol Sci. 2020. PMID: 32751106 Free PMC article.
-
Detection of COVID-19: A review of the current literature and future perspectives.Biosens Bioelectron. 2020 Oct 15;166:112455. doi: 10.1016/j.bios.2020.112455. Epub 2020 Jul 21. Biosens Bioelectron. 2020. PMID: 32739797 Free PMC article. Review.
-
Point-of-Care Diagnostics of COVID-19: From Current Work to Future Perspectives.Sensors (Basel). 2020 Jul 31;20(15):4289. doi: 10.3390/s20154289. Sensors (Basel). 2020. PMID: 32752043 Free PMC article. Review.
Cited by
-
Rapid Large-Scale COVID-19 Testing During Shortages.Diagnostics (Basel). 2020 Jul 8;10(7):464. doi: 10.3390/diagnostics10070464. Diagnostics (Basel). 2020. PMID: 32650631 Free PMC article.
-
Development of loop-mediated isothermal method and comparison with conventional PCR assay for rapid on spot identification of tissue of cattle origin.J Food Sci Technol. 2021 Dec;58(12):4608-4615. doi: 10.1007/s13197-020-04948-8. Epub 2021 Jan 5. J Food Sci Technol. 2021. PMID: 34629525 Free PMC article.
-
Uncovering career alternatives amidst the COVID-19 pandemic: a medical student's perspective.Public Health. 2021 Nov;200:e8-e9. doi: 10.1016/j.puhe.2021.09.020. Epub 2021 Sep 25. Public Health. 2021. PMID: 34702573 Free PMC article. No abstract available.
-
Rapid Detection of Severe Fever with Thrombocytopenia Syndrome Virus in the Acute Phase of Infection by Direct Real-Time Reverse Transcription without RNA Extraction.Am J Trop Med Hyg. 2024 Jun 18;111(2):429-432. doi: 10.4269/ajtmh.23-0541. Print 2024 Aug 7. Am J Trop Med Hyg. 2024. PMID: 38889707 Free PMC article.
-
Evaluation of six different rapid methods for nucleic acid detection of SARS-COV-2 virus.J Med Virol. 2021 Sep;93(9):5538-5543. doi: 10.1002/jmv.27090. Epub 2021 Jun 8. J Med Virol. 2021. PMID: 34002401 Free PMC article.
References
-
- WHO WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. [(accessed on 12 March 2020)]; Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re....
-
- WHO COVID-19 Dashboard. [(accessed on 16 April 2020)]; Available online: https://covid19.who.int/
-
- Ng Y., Li Z., Chua Y.X., Chaw W.L., Zhao Z., Er B., Pung R., Chiew C.J., Lye D.C., Heng D., et al. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore — January 2–February 29, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020;69:307–311. doi: 10.15585/mmwr.mm6911e1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

