Epigenetic Regulation of the Human Papillomavirus Life Cycle
- PMID: 32570816
- PMCID: PMC7350343
- DOI: 10.3390/pathogens9060483
Epigenetic Regulation of the Human Papillomavirus Life Cycle
Abstract
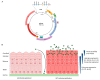
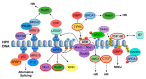
Persistent infection with certain types of human papillomaviruses (HPVs), termed high risk, presents a public health burden due to their association with multiple human cancers, including cervical cancer and an increasing number of head and neck cancers. Despite the development of prophylactic vaccines, the incidence of HPV-associated cancers remains high. In addition, no vaccine has yet been licensed for therapeutic use against pre-existing HPV infections and HPV-associated diseases. Although persistent HPV infection is the major risk factor for cancer development, additional genetic and epigenetic alterations are required for progression to the malignant phenotype. Unlike genetic mutations, the reversibility of epigenetic modifications makes epigenetic regulators ideal therapeutic targets for cancer therapy. This review article will highlight the recent advances in the understanding of epigenetic modifications associated with HPV infections, with a particular focus on the role of these epigenetic changes during different stages of the HPV life cycle that are closely associated with activation of DNA damage response pathways.
Keywords: DNA damage response; DNA repair; HPV; epigenetics; histone; life cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
HPV: Molecular pathways and targets.Curr Probl Cancer. 2018 Mar-Apr;42(2):161-174. doi: 10.1016/j.currproblcancer.2018.03.003. Epub 2018 Apr 5. Curr Probl Cancer. 2018. PMID: 29706467 Review.
-
The role of HPV-induced epigenetic changes in cervical carcinogenesis (Review).Biomed Rep. 2021 Jul;15(1):60. doi: 10.3892/br.2021.1436. Epub 2021 May 20. Biomed Rep. 2021. PMID: 34094536 Free PMC article. Review.
-
FANCD2 Binds Human Papillomavirus Genomes and Associates with a Distinct Set of DNA Repair Proteins to Regulate Viral Replication.mBio. 2017 Feb 14;8(1):e02340-16. doi: 10.1128/mBio.02340-16. mBio. 2017. PMID: 28196964 Free PMC article.
-
Epigenetic Alterations in Human Papillomavirus-Associated Cancers.Viruses. 2017 Sep 1;9(9):248. doi: 10.3390/v9090248. Viruses. 2017. PMID: 28862667 Free PMC article. Review.
-
Therapeutic Vaccines Against Human Papilloma Viruses: Achievements and Prospects.Biochemistry (Mosc). 2019 Jul;84(7):800-816. doi: 10.1134/S0006297919070101. Biochemistry (Mosc). 2019. PMID: 31509730 Review.
Cited by
-
Human papillomavirus-related neoplasia of the ocular adnexa.Acta Ophthalmol. 2022 Oct;100 Suppl 272(Suppl 272):3-33. doi: 10.1111/aos.15244. Acta Ophthalmol. 2022. PMID: 36203222 Free PMC article.
-
siRNA-Inhibition of TIGAR Hypersensitizes Human Papillomavirus-Transformed Cells to Apoptosis Induced by Chemotherapy Drugs that Cause Oxidative Stress.J Antivir Antiretrovir. 2021;13(4):223. Epub 2021 May 31. J Antivir Antiretrovir. 2021. PMID: 35291688 Free PMC article.
-
Peptide-Based Nanovaccines in the Treatment of Cervical Cancer: A Review of Recent Advances.Int J Nanomedicine. 2022 Feb 25;17:869-900. doi: 10.2147/IJN.S269986. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35241913 Free PMC article. Review.
-
Effect of HPV Oncoprotein on Carbohydrate and Lipid Metabolism in Tumor Cells.Curr Cancer Drug Targets. 2024;24(10):987-1004. doi: 10.2174/0115680096266981231215111109. Curr Cancer Drug Targets. 2024. PMID: 38284713 Review.
-
HPV pathogenesis, various types of vaccines, safety concern, prophylactic and therapeutic applications to control cervical cancer, and future perspective.Virusdisease. 2023 May 24;34(2):1-19. doi: 10.1007/s13337-023-00824-z. Online ahead of print. Virusdisease. 2023. PMID: 37363362 Free PMC article. Review.
References
-
- PaVE: Papilloma Virus Genome Database. [(accessed on 6 January 2020)]; Available online: https://pave.niaid.nih.gov/
-
- Walboomers J.M.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J.F., Peto J., Meijer C.J.L.M., Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

