How and Why Are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH
- PMID: 32570870
- PMCID: PMC7352839
- DOI: 10.3390/cancers12061616
How and Why Are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH
Abstract
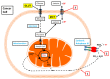
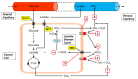
The acidic tumour microenvironment is now recognized as a tumour phenotype that drives cancer somatic evolution and disease progression, causing cancer cells to become more invasive and to metastasise. This property of solid tumours reflects a complex interplay between cellular carbon metabolism and acid removal that is mediated by cell membrane carbonic anhydrases and various transport proteins, interstitial fluid buffering, and abnormal tumour-associated vessels. In the past two decades, a convergence of advances in the experimental and mathematical modelling of human cancers, as well as non-invasive pH-imaging techniques, has yielded new insights into the physiological mechanisms that govern tumour extracellular pH (pHe). In this review, we examine the mechanisms by which solid tumours maintain a low pHe, with a focus on carbonic anhydrase IX (CAIX), a cancer-associated cell surface enzyme. We also review the accumulating evidence that suggest a role for CAIX as a biological pH-stat by which solid tumours stabilize their pHe. Finally, we highlight the prospects for the clinical translation of CAIX-targeted therapies in oncology.
Keywords: cancer metabolism; cancer microenvironment; carbonic anhydrase IX; magnetic resonance spectroscopy; models of tumour pH regulation; pH measurement in vivo; pH-stat; tumour pH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Warburg O., Dickens F., Wilhelm K. The Metabolism of Tumours: Investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlem. Arnold Constable; London, UK: 1930.
-
- Griffiths J.R., McIntyre D.J., Howe F.A., Stubbs M. Why are cancers acidic? A carrier-mediated diffusion model for H+ transport in the interstitial fluid. Novartis Found. Symp. 2001;240:46–62. discussion 62–67, 152–153. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

