Hepatitis B Virus Entry into Cells
- PMID: 32570893
- PMCID: PMC7349259
- DOI: 10.3390/cells9061486
Hepatitis B Virus Entry into Cells
Abstract
Hepatitis B virus (HBV), an enveloped partially double-stranded DNA virus, is a widespread human pathogen responsible for more than 250 million chronic infections worldwide. Current therapeutic strategies cannot eradicate HBV due to the persistence of the viral genome in a special DNA structure (covalently closed circular DNA, cccDNA). The identification of sodium taurocholate co-transporting polypeptide (NTCP) as an entry receptor for both HBV and its satellite virus hepatitis delta virus (HDV) has led to great advances in our understanding of the life cycle of HBV, including the early steps of infection in particular. However, the mechanisms of HBV internalization and the host factors involved in this uptake remain unclear. Improvements in our understanding of HBV entry would facilitate the design of new therapeutic approaches targeting this stage and preventing the de novo infection of naïve hepatocytes. In this review, we provide an overview of current knowledge about the process of HBV internalization into cells.
Keywords: Hepatitis B virus; entry pathway; virus–host interaction.
Conflict of interest statement
The authors have no conflicts of interest to declare.
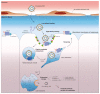
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

