Empowering the Medicinal Applications of Bisphosphonates by Unveiling their Synthesis Details
- PMID: 32570958
- PMCID: PMC7356784
- DOI: 10.3390/molecules25122821
Empowering the Medicinal Applications of Bisphosphonates by Unveiling their Synthesis Details
Abstract
Bisphosphonates (BPs), well-known medicinal compounds used for osteoporosis management, are currently the target of intensive research, from basic pre-formulation studies to more advanced stages of clinical practice. The high demand by the pharmaceutical industry inherently requires an easy, efficient and quick preparation of BPs. Current synthetic procedures are, however, still far from ideal. This work presents a comprehensive compilation of reports on the synthesis of the commercially available bisphosphonates that are pharmaceutical active ingredients. Current limitations to the conventional synthesis are assessed, and paths towards their improvement are described, either through the use of alternative solvents and/or by selecting appropriate ratios of the reactants. Innovative processes, such as microwave-assisted synthesis, are presented as more environmental-friendly and effective methods. The main advantages and setbacks of all syntheses are provided as a way to clarify and promote the development of simpler and improved procedures. Only in this way one will be able to efficiently respond to the future high demand of BPs, mostly due to the increase in life span in occidental countries.
Keywords: P-reactants; bisphosphonates; pharmaceutical applications; reactional solvents; research-driven studies; synthesis improvement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


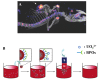

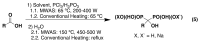
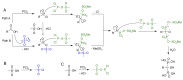
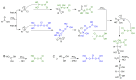
References
-
- U.S. Food and Drug Administration Drugs@FDA: FDA Approved Drug Products. [(accessed on 2 June 2020)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
-
- Keglevich G., Grün A., Aradi K., Garadnay S., Greiner I. Optimized synthesis of N-heterocyclic dronic acids; closing a black-box era. Tetrahedron Lett. 2011;52:2744–2746. doi: 10.1016/j.tetlet.2011.03.093. - DOI
-
- Keglevich G., Grün A., Garadnay S., Greiner I. Rational synthesis of dronic acid derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2015;190:2116–2124. doi: 10.1080/10426507.2015.1072194. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

