A lineage-tracing tool to map the fate of hypoxic tumour cells
- PMID: 32571767
- PMCID: PMC7406318
- DOI: 10.1242/dmm.044768
A lineage-tracing tool to map the fate of hypoxic tumour cells
Abstract
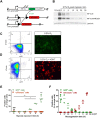
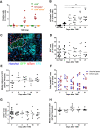
Intratumoural hypoxia is a common characteristic of malignant treatment-resistant cancers. However, hypoxia-modification strategies for the clinic remain elusive. To date, little is known on the behaviour of individual hypoxic tumour cells in their microenvironment. To explore this issue in a spatial and temporally controlled manner, we developed a genetically encoded sensor by fusing the O2-labile hypoxia-inducible factor 1α (HIF-1α) protein to eGFP and a tamoxifen-regulated Cre recombinase. Under normoxic conditions, HIF-1α is degraded but, under hypoxia, the HIF-1α-GFP-Cre-ERT2 fusion protein is stabilised and in the presence of tamoxifen activates a tdTomato reporter gene that is constitutively expressed in hypoxic progeny. We visualise the random distribution of hypoxic tumour cells from hypoxic or necrotic regions and vascularised areas using immunofluorescence and intravital microscopy. Once tdTomato expression is induced, it is stable for at least 4 weeks. Using this system, we could show in vivo that the post-hypoxic cells were more proliferative than non-labelled cells. Our results demonstrate that single-cell lineage tracing of hypoxic tumour cells can allow visualisation of their behaviour in living tumours using intravital microscopy. This tool should prove valuable for the study of dissemination and treatment response of post-hypoxic tumour cells in vivo at single-cell resolution.This article has an associated First Person interview with the joint first authors of the paper.
Keywords: Cre recombinase; HIF; Hypoxia; Intravital imaging; Lineage tracing; NSCLC tumour.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Conway J. R. W., Warren S. C., Herrmann D., Murphy K. J., Cazet A. S., Vennin C. and Timpson P. (2018). Intravital Imaging to Monitor Therapeutic Response in Moving Hypoxic Regions Resistant to PI3K Pathway Targeting in Pancreatic Cancer. Cell Rep. 23, 3312-3326. 10.1016/j.celrep.2018.05.038 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

