BCALM (AC099524.1) Is a Human B Lymphocyte-Specific Long Noncoding RNA That Modulates B Cell Receptor-Mediated Calcium Signaling
- PMID: 32571842
- PMCID: PMC7372127
- DOI: 10.4049/jimmunol.2000088
BCALM (AC099524.1) Is a Human B Lymphocyte-Specific Long Noncoding RNA That Modulates B Cell Receptor-Mediated Calcium Signaling
Abstract
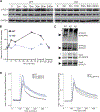
Of the thousands of long noncoding RNAs (lncRNA) identified in lymphocytes, very few have defined functions. In this study, we report the discovery and functional elucidation of a human B cell-specific lncRNA with high levels of expression in three types of B cell cancer and normal B cells. The AC099524.1 gene is upstream of the gene encoding the B cell-specific phospholipase C γ 2 (PLCG2), a B cell-specific enzyme that stimulates intracellular Ca2+ signaling in response to BCR activation. AC099524.1 (B cell-associated lncRNA modulator of BCR-mediated Ca+ signaling [BCALM]) transcripts are localized in the cytoplasm and, as expected, CRISPR/Cas9 knockout of AC099524.1 did not affect PLCG2 mRNA or protein expression. lncRNA interactome, RNA immunoprecipitation, and coimmunoprecipitation studies identified BCALM-interacting proteins in B cells, including phospholipase D 1 (PLD1), and kinase adaptor proteins AKAP9 (AKAP450) and AKAP13 (AKAP-Lbc). These two AKAP proteins form signaling complexes containing protein kinases A and C, which phosphorylate and activate PLD1 to produce phosphatidic acid (PA). BCR stimulation of BCALM-deficient B cells resulted in decreased PLD1 phosphorylation and increased intracellular Ca+ flux relative to wild-type cells. These results suggest that BCALM promotes negative feedback that downmodulates BCR-mediated Ca+ signaling by promoting phosphorylation of PLD1 by AKAP-associated kinases, enhancing production of PA. PA activates SHP-1, which negatively regulates BCR signaling. We propose the name BCALM for B-Cell Associated LncRNA Modulator of BCR-mediated Ca+ signaling. Our findings suggest a new, to our knowledge, paradigm for lncRNA-mediated modulation of lymphocyte activation and signaling, with implications for B cell immune response and BCR-dependent cancers.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures




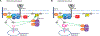
References
-
- Koues OI, Kowalewski RA, Chang L-W, Pyfrom SC, Schmidt JA, Luo H, Sandoval LE, Hughes TB, Bednarski JJ, Cashen AF, Payton JE, and Oltz EM. 2015. Enhancer sequence variants and transcription-factor deregulation synergize to construct pathogenic regulatory circuits in B-cell lymphoma. Immunity 42: 186–198. - PMC - PubMed
-
- Huarte M 2015. The emerging role of lncRNAs in cancer. Nat. Med. 21: 1253–1261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

