Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development
- PMID: 32571934
- PMCID: PMC7368255
- DOI: 10.1073/pnas.2009799117
Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development
Abstract
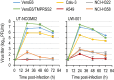
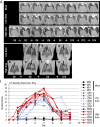
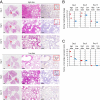
At the end of 2019, a novel coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2) was detected in Wuhan, China, that spread rapidly around the world, with severe consequences for human health and the global economy. Here, we assessed the replicative ability and pathogenesis of SARS-CoV-2 isolates in Syrian hamsters. SARS-CoV-2 isolates replicated efficiently in the lungs of hamsters, causing severe pathological lung lesions following intranasal infection. In addition, microcomputed tomographic imaging revealed severe lung injury that shared characteristics with SARS-CoV-2-infected human lung, including severe, bilateral, peripherally distributed, multilobular ground glass opacity, and regions of lung consolidation. SARS-CoV-2-infected hamsters mounted neutralizing antibody responses and were protected against subsequent rechallenge with SARS-CoV-2. Moreover, passive transfer of convalescent serum to naïve hamsters efficiently suppressed the replication of the virus in the lungs even when the serum was administrated 2 d postinfection of the serum-treated hamsters. Collectively, these findings demonstrate that this Syrian hamster model will be useful for understanding SARS-CoV-2 pathogenesis and testing vaccines and antiviral drugs.
Keywords: SARS-CoV-2; Syrian hamsters; countermeasure; infection.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

