Synthesis of diglycolic acid functionalized core-shell silica coated Fe3O4 nanomaterials for magnetic extraction of Pb(II) and Cr(VI) ions
- PMID: 32572117
- PMCID: PMC7308298
- DOI: 10.1038/s41598-020-67168-2
Synthesis of diglycolic acid functionalized core-shell silica coated Fe3O4 nanomaterials for magnetic extraction of Pb(II) and Cr(VI) ions
Abstract
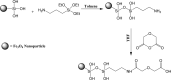
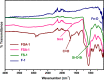
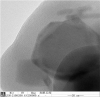
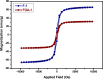
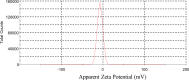
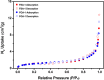
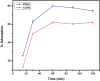
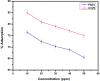
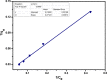
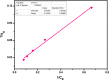
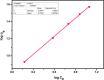
Amine-terminated core-shell silica coated magnetite nanoparticles were functionalized with diglycolic acid for the first time to create acid moiety on the surface of the nanoparticles. The formation of magnetite nanoparticles was scrutinised through XRD, SEM, EDS, TEM, VSM and FTIR spectroscopy. The BET surface area of nano-sorbent was found to be 4.04 m2/g with pore size 23.68 nm. These nanomaterials were then utilized to remove the Pb(II) and Cr(VI) ions from their aqueous media and uptake of metal ions was determined by atomic absorption spectroscopy (AAS). A batch adsorption technique was applied to remove both ions at optimised pH and contact time with maximum adsorption efficiency for Pb(II) ions at pH 7 while for Cr(VI) ions at pH 3. Adsorption mechanism was studied using Langmuir and Freundlich isotherms and equilibrium data fitted well for both the isotherms, showing complex nature of adsorption comprising both chemisorption as well as physio-sorption phenomena. The nanosorbents exhibited facile separation by applying external magnetic field due to the ferrimagnetic behaviour with 31.65 emu/g saturation magnetization. These nanosorbents were also found to be used multiple times after regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Xu P, et al. Adsorption of Pb(II) by iron oxide nanoparticles immobilized phanerochaete chrysosporium: Equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem. Eng. J. 2012;203:423–431. doi: 10.1016/j.cej.2012.07.048. - DOI
-
- Wang H, et al. Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr(VI) removal. Chem. Eng. J. 2015;262:597–606. doi: 10.1016/j.cej.2014.10.020. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

