Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study
- PMID: 32572189
- PMCID: PMC7387243
- DOI: 10.1038/s41375-020-0915-9
Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study
Abstract
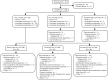
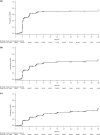
Bosutinib is approved for newly diagnosed Philadelphia chromosome-positive (Ph+) chronic phase (CP) chronic myeloid leukemia (CML) and for Ph+ CP, accelerated (AP), or blast (BP) phase CML after prior treatment with tyrosine kinase inhibitors (TKIs). In the ongoing phase 4 BYOND study (NCT02228382), 163 CML patients resistant/intolerant to prior TKIs (n = 156 Ph+ CP CML, n = 4 Ph+ AP CML, n = 3 Ph-negative/BCR-ABL1+ CML) received bosutinib 500 mg once daily (starting dose). As of ≥1 year after last enrolled patient (median treatment duration 23.7 months), 56.4% of Ph+ CP CML patients remained on bosutinib. Primary endpoint of cumulative confirmed major cytogenetic response (MCyR) rate by 1 year was 75.8% in Ph+ CP CML patients after one or two prior TKIs and 62.2% after three prior TKIs. Cumulative complete cytogenetic response (CCyR) and major molecular response (MMR) rates by 1 year were 80.6% and 70.5%, respectively, in Ph+ CP CML patients overall. No patient progressed to AP/BP on treatment. Across all patients, the most common treatment-emergent adverse events were diarrhea (87.7%), nausea (39.9%), and vomiting (32.5%). The majority of patients had confirmed MCyR by 1 year and MMR by 1 year, further supporting bosutinib use for Ph+ CP CML patients resistant/intolerant to prior TKIs.
Conflict of interest statement
Authors declare the following potential conflicts of interest: AH received honoraria from BMS, Novartis, Pfizer, and Takeda, and received research support from BMS, Incyte, Novartis, and Pfizer. CGP provides consultancy to BMS and received honoraria and research support from Pfizer. CA serves on the advisory board for Archer DX, Jazz Pharma, and Tetraphase Pharma, provides consultancy to Actinium, Agios, Bayer, Cardinal Health, Gerson Lehman Group, Incyte, Jazz Pharma, NKarta, Pfizer, Seattle Genetics, and Tetraphase Pharma, received honoraria from Agios, Cardinal Health, and Jazz Pharma, received research support from Pfizer, and serves on the speakers bureau for Jazz Pharma / provides expert testimony for Dava Oncology. BTG is a consultant for BerGenBio, Novartis, Pfizer, and Sanofi Genzyme, received research support from Pfizer, and has stock ownership in Alden Cancer Therapy AS and KinN Therapeutics AS. THB is a consultant for Janssen, Merck, Novartis, Pfizer, and Takeda, and received research support from Novartis and Pfizer. BDS received honoraria for consulting to Agios, Celgene, Jazz Pharma, Novartis and Pfizer, and received research support from Pfizer. PGC received research support from BMS and Pfizer. UOS received research support from Pfizer. SS received honoraria from BMS, Incyte, Novartis, and Pfizer, and received research support from BMS, Incyte, Novartis, and Pfizer. TE received research support from BMS, Incyte, Novartis, and Pfizer. NBB, AV, EL, ARS and JL are employees of Pfizer. GR received research support from Pfizer and served on the speaker bureau for BMS, Incyte, Novartis, and Pfizer. JW serves on the advisory board for Jazz Pharma and Takeda, received research support from Pfizer and Takeda, and serves on the data monitoring committee for Rafael Pharma. FJG is a consultant to Actuate Therapeutics Inc, provides expert testimony to Novartis, and received research support from Pfizer.
Figures

References
-
- National Cancer Institute. Chronic Myelogenous Leukemia Treatment (PDQ®). 2020. https://www.cancer.gov/types/leukemia/hp/cml-treatment-pdq#_1
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

