Tau molecular diversity contributes to clinical heterogeneity in Alzheimer's disease
- PMID: 32572268
- PMCID: PMC7603860
- DOI: 10.1038/s41591-020-0938-9
Tau molecular diversity contributes to clinical heterogeneity in Alzheimer's disease
Erratum in
-
Author Correction: Tau molecular diversity contributes to clinical heterogeneity in Alzheimer's disease.Nat Med. 2021 Feb;27(2):356. doi: 10.1038/s41591-021-01251-7. Nat Med. 2021. PMID: 33514949 Free PMC article. No abstract available.
Abstract
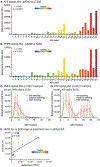
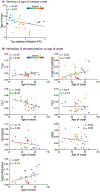
Alzheimer's disease (AD) causes unrelenting, progressive cognitive impairments, but its course is heterogeneous, with a broad range of rates of cognitive decline1. The spread of tau aggregates (neurofibrillary tangles) across the cerebral cortex parallels symptom severity2,3. We hypothesized that the kinetics of tau spread may vary if the properties of the propagating tau proteins vary across individuals. We carried out biochemical, biophysical, MS and both cell- and animal-based-bioactivity assays to characterize tau in 32 patients with AD. We found striking patient-to-patient heterogeneity in the hyperphosphorylated species of soluble, oligomeric, seed-competent tau. Tau seeding activity correlates with the aggressiveness of the clinical disease, and some post-translational modification (PTM) sites appear to be associated with both enhanced seeding activity and worse clinical outcomes, whereas others are not. These data suggest that different individuals with 'typical' AD may have distinct biochemical features of tau. These data are consistent with the possibility that individuals with AD, much like people with cancer, may have multiple molecular drivers of an otherwise common phenotype, and emphasize the potential for personalized therapeutic approaches for slowing clinical progression of AD.
Conflict of interest statement
Competing interests
This work was supported in part by a research agreement to Massachusetts General Hospital from Merck & Co. D.J.-G, R.C., R.M., K.A., F.E., and M.E.K. are/were full time employees of Merck & Co. Inc. during the course of the work.
Figures












References
-
- Giannakopoulos P et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60, 1495–1500 (2003). - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET & Hyman BT Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AG064039/AG/NIA NIH HHS/United States
- 1RF1AG059789/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)/International
- RF1 AG058674/AG/NIA NIH HHS/United States
- 1K08AG064039/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)/International
- 2018-AARF-591935/Alzheimer's Association/International
- R56 AG061196/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- 1P30AG062421/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)/International
- P50 AG005134/AG/NIA NIH HHS/United States
- RF1 AG059789/AG/NIA NIH HHS/United States
- 1RF1AG058674/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)/International
- P2ELP3_184403/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

