Laboratory considerations amidst the coronavirus disease 2019 outbreak: the Spedali Civili in Brescia experience
- PMID: 32573254
- PMCID: PMC7315825
- DOI: 10.4155/bio-2020-0109
Laboratory considerations amidst the coronavirus disease 2019 outbreak: the Spedali Civili in Brescia experience
Abstract
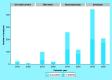
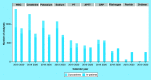
Coronavirus disease 2019 emergency has created an enormous stress on providers that have been transformed into coronavirus disease hospitals. This article presents the experience of the clinical laboratory of Spedali Civili in Brescia (a teaching hospital in Lombardy with over 1500 beds) in managing the crisis, and to offer practical considerations for laboratory testing for this cohort of patients. Our contribution is threefold: by comparing the demand for tests in two representative period before and within the crisis, we show the change in compositions of the analytes that other labs may expect; we present the new panels of tests that hospital staff can order with different advantage for wards and laboratory; and we show how to reorganize staff on the basis of changes mentioned above.
Keywords: COVID hospital; COVID-19 emergency; lab organization; panels for monitoring patients; reagents; staff management.
Figures
References
-
- Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. (2020) (Epub ahead of print). - PubMed
-
• Explains the peculiariaties of coronavirus disease 2019 (COVID-19) patients from the point of view of clinical labs. An instrument for diagnosis.
-
- Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA (2020) (Epub ahead of print). - PubMed
-
- Tan SS, Yan B, Saw S. et al. Practical laboratory considerations amidst the COVID-19 outbreak: early experience from Singapore. J. Clin. Pathol. (2020) (Epub ahead of print). - PubMed
-
• Explains how labs in Singapore have responded to the crisis, especially in terms of time turnaround and test required.
-
- Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. (2020) (Epub ahead of print). - PubMed
-
• Explains how labs may help for the diagnosis and treatment of COVID-19 patients.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous