Trends and Sex Differences in Access to HIV Care with Scale Up of National HIV Treatment Guidelines in Pune, India
- PMID: 32573318
- PMCID: PMC7313325
- DOI: 10.1177/2325958220931735
Trends and Sex Differences in Access to HIV Care with Scale Up of National HIV Treatment Guidelines in Pune, India
Abstract
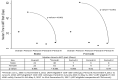
Test and treat is the current global standard, yet sex differences persist in access to HIV care. We assessed the differences in presentation and antiretroviral therapy (ART) uptake by sex and ART-eligibility period among ART-naive adults registered at a public ART center in India. Four ART eligibility periods were defined by programmatically determined CD4 criteria (periods I-IV: CD4 <200, <350, ≤500 cells/μL, and any CD4) between January 2005 and December 2017. Of 23 957 participants, 12 510 were male. Men consistently presented with lower median CD4 count (period I-IV, P < .05) and higher median age (period I-III, P < .001) than women. From period I to IV, median age increased in women (P < .0001), ART initiation time decreased in both sexes (P < .001), and median CD4 remained <200 cells/µL in men. Advanced HIV disease and increasing age at presentation are persistent sex-specific trends which warrant innovative HIV testing strategies in both sexes.
Keywords: HIV/AIDS; India; age at presentation; antiretroviral therapy guidelines; sex differences.
Conflict of interest statement
Figures


References
-
- TEMPRANO ANRS 12136 Study Group; Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi:10.1056/NEJMoa1507198 - PubMed
-
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Published 2015. Accessed December 26, 2018 https://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. WHO Guidel. 2013;272 ISBN: 978 92 4 150572 7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

