Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism
- PMID: 32573437
- PMCID: PMC7311170
- DOI: 10.7554/eLife.56185
Moyamoya disease factor RNF213 is a giant E3 ligase with a dynein-like core and a distinct ubiquitin-transfer mechanism
Abstract
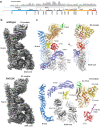
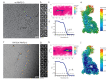
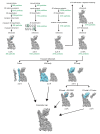
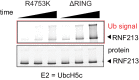
RNF213 is the major susceptibility factor for Moyamoya disease, a progressive cerebrovascular disorder that often leads to brain stroke in adults and children. Characterization of disease-associated mutations has been complicated by the enormous size of RNF213. Here, we present the cryo-EM structure of mouse RNF213. The structure reveals the intricate fold of the 584 kDa protein, comprising an N-terminal stalk, a dynein-like core with six ATPase units, and a multidomain E3 module. Collaboration with UbcH7, a cysteine-reactive E2, points to an unexplored ubiquitin-transfer mechanism that proceeds in a RING-independent manner. Moreover, we show that pathologic MMD mutations cluster in the composite E3 domain, likely interfering with substrate ubiquitination. In conclusion, the structure of RNF213 uncovers a distinct type of an E3 enzyme, highlighting the growing mechanistic diversity in ubiquitination cascades. Our results also provide the molecular framework for investigating the emerging role of RNF213 in lipid metabolism, hypoxia, and angiogenesis.
Keywords: biochemistry; cerebrovascular disorder; chemical biology; human; molecular biophysics; molecular machines; mouse; protein quality control; protein ubiquitination; rare disease; structural biology.
Plain language summary
Moyamoya disease is a genetic disorder affecting both adults and children. It is characterized by narrowing of the blood vessels in the brain, which can lead to strokes. Moyamoya patients often have mutations in the gene for a protein called RNF213. This protein is linked to multiple processes in the body, including the development of blood vessels. Despite this, its role in Moyamoya disease is still something of a mystery. RNF213 is known to fall into two protein ‘classes’. First, it is an E3 enzyme. This type of protein tags unwanted or defective proteins for disposal by the cell. Second, it is a motor protein. Motor proteins contain tiny molecular ‘engines’, called ATPases, that normally convert chemical energy to movement. No other human protein combines these two activities, making RNF213 unique. RNF213 is also an extremely large protein, which means it is difficult to manipulate in the laboratory and thus hard to study. Scientists still need more detailed information on RNF213’s structure and chemical activity before we can understand what the mutant protein might be doing in Moyamoya disease. Ahel et al. therefore set out to make the RNF213 protein and ‘dissect’ it in a test tube. Electron microscopy experiments using the mouse-version of RNF213 revealed that it consisted of a single, giant molecule, folded up to form three regions with distinct structures. These were a long ‘arm’ at one end, a ring-shaped part in the middle, containing the ATPase ‘motor’, and the E3 enzyme module at the other end. Further chemical analysis showed that RNF213’s ATPase and E3 modules worked in unexpected ways. Although the ATPase did resemble another well-known motor protein, in RNF213 it did not generate movement but rather appeared to act like an intricate molecular ‘switch’. The E3 module of RNF213 ‘tagged’ other molecules as expected but did not contain an additional structure that all other known E3 enzymes need to work properly. This suggests that RNF213 represents a distinct class of E3 enzymes. Biochemical tests of the mutation most commonly found in Moyamoya patients revealed that it left RNF213’s overall structure, ATPase motor and E3 module intact. That is, the disease-causing mutation appeared to hinder interactions with other partner proteins, rather than disrupting RNF213 itself. By providing the first detailed molecular description of the architecture of RNF213, Ahel et al. hope that these findings will help future investigations of both this giant protein’s biological role in the cell and its contribution to Moyamoya disease.
© 2020, Ahel et al.
Conflict of interest statement
JA, AL, AV, AS, AM, DH, TC No competing interests declared
Figures










References
-
- Ahel J. ClausenLab-ViennaBioCenter; 2020. https://github.com/ClausenLab-ViennaBioCenter/CrossLinkingVisualizer
-
- Banh RS, Iorio C, Marcotte R, Xu Y, Cojocari D, Rahman AA, Pawling J, Zhang W, Sinha A, Rose CM, Isasa M, Zhang S, Wu R, Virtanen C, Hitomi T, Habu T, Sidhu SS, Koizumi A, Wilkins SE, Kislinger T, Gygi SP, Schofield CJ, Dennis JW, Wouters BG, Neel BG. PTP1B controls non-mitochondrial oxygen consumption by regulating RNF213 to promote tumour survival during hypoxia. Nature Cell Biology. 2016;18:803–813. doi: 10.1038/ncb3376. - DOI - PMC - PubMed

