Skd3 (human ClpB) is a potent mitochondrial protein disaggregase that is inactivated by 3-methylglutaconic aciduria-linked mutations
- PMID: 32573439
- PMCID: PMC7343390
- DOI: 10.7554/eLife.55279
Skd3 (human ClpB) is a potent mitochondrial protein disaggregase that is inactivated by 3-methylglutaconic aciduria-linked mutations
Abstract
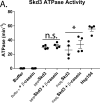
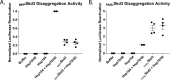
Cells have evolved specialized protein disaggregases to reverse toxic protein aggregation and restore protein functionality. In nonmetazoan eukaryotes, the AAA+ disaggregase Hsp78 resolubilizes and reactivates proteins in mitochondria. Curiously, metazoa lack Hsp78. Hence, whether metazoan mitochondria reactivate aggregated proteins is unknown. Here, we establish that a mitochondrial AAA+ protein, Skd3 (human ClpB), couples ATP hydrolysis to protein disaggregation and reactivation. The Skd3 ankyrin-repeat domain combines with conserved AAA+ elements to enable stand-alone disaggregase activity. A mitochondrial inner-membrane protease, PARL, removes an autoinhibitory peptide from Skd3 to greatly enhance disaggregase activity. Indeed, PARL-activated Skd3 solubilizes α-synuclein fibrils connected to Parkinson's disease. Human cells lacking Skd3 exhibit reduced solubility of various mitochondrial proteins, including anti-apoptotic Hax1. Importantly, Skd3 variants linked to 3-methylglutaconic aciduria, a severe mitochondrial disorder, display diminished disaggregase activity (but not always reduced ATPase activity), which predicts disease severity. Thus, Skd3 is a potent protein disaggregase critical for human health.
Keywords: AAA+ protein; Hsp104; Hsp78; Skd3; biochemistry; chemical biology; disaggregase; human; protein misfolding.
© 2020, Cupo and Shorter.
Conflict of interest statement
RC, JS No competing interests declared
Figures














References
-
- Angebault C, Charif M, Guegen N, Piro-Megy C, Mousson de Camaret B, Procaccio V, Guichet PO, Hebrard M, Manes G, Leboucq N, Rivier F, Hamel CP, Lenaers G, Roubertie A. Mutation in NDUFA13/GRIM19 leads to early onset hypotonia, dyskinesia and sensorial deficiencies, and mitochondrial complex I instability. Human Molecular Genetics. 2015;24:3948–3955. doi: 10.1093/hmg/ddv133. - DOI - PubMed
-
- Anunciado-Koza RP, Zhang J, Ukropec J, Bajpeyi S, Koza RA, Rogers RC, Cefalu WT, Mynatt RL, Kozak LP. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. The Journal of Biological Chemistry. 2011;286:11659–11671. doi: 10.1074/jbc.M110.203000. - DOI - PMC - PubMed
-
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology the gene ontology consortium. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. - DOI - PMC - PubMed
-
- Bateman JM, Iacovino M, Perlman PS, Butow RA. Mitochondrial DNA instability mutants of the bifunctional protein Ilv5p have altered organization in mitochondria and are targeted for degradation by Hsp78 and the Pim1p protease. The Journal of Biological Chemistry. 2002;277:47946–47953. doi: 10.1074/jbc.M209071200. - DOI - PubMed
-
- Baumann U, Fernández-Sáiz V, Rudelius M, Lemeer S, Rad R, Knorn AM, Slawska J, Engel K, Jeremias I, Li Z, Tomiatti V, Illert AL, Targosz BS, Braun M, Perner S, Leitges M, Klapper W, Dreyling M, Miething C, Lenz G, Rosenwald A, Peschel C, Keller U, Kuster B, Bassermann F. Disruption of the PRKCD-FBXO25-HAX-1 Axis attenuates the apoptotic response and drives lymphomagenesis. Nature Medicine. 2014;20:1401–1409. doi: 10.1038/nm.3740. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

