Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases
- PMID: 32573694
- PMCID: PMC7478735
- DOI: 10.1084/jem.20191430
Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases
Abstract
Chronic pain is a major comorbidity of chronic inflammatory diseases. Here, we report that the cytokine IL-1β, which is abundantly produced during multiple sclerosis (MS), arthritis (RA), and osteoarthritis (OA) both in humans and in animal models, drives pain associated with these diseases. We found that the type 1 IL-1 receptor (IL-1R1) is highly expressed in the mouse and human by a subpopulation of TRPV1+ dorsal root ganglion neurons specialized in detecting painful stimuli, termed nociceptors. Strikingly, deletion of the Il1r1 gene specifically in TRPV1+ nociceptors prevented the development of mechanical allodynia without affecting clinical signs and disease progression in mice with experimental autoimmune encephalomyelitis and K/BxN serum transfer-induced RA. Conditional restoration of IL-1R1 expression in nociceptors of IL-1R1-knockout mice induced pain behavior but did not affect joint damage in monosodium iodoacetate-induced OA. Collectively, these data reveal that neuronal IL-1R1 signaling mediates pain, uncovering the potential benefit of anti-IL-1 therapies for pain management in patients with chronic inflammatory diseases.
© 2020 Mailhot et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
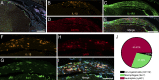

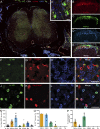
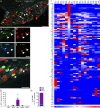


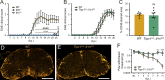



References
-
- Arima Y., Kamimura D., Atsumi T., Harada M., Kawamoto T., Nishikawa N., Stofkova A., Ohki T., Higuchi K., Morimoto Y., et al. . 2015. A pain-mediated neural signal induces relapse in murine autoimmune encephalomyelitis, a multiple sclerosis model. eLife. 4 e08733 10.7554/eLife.08733 - DOI - PMC - PubMed
-
- Aubé B., Lévesque S.A., Paré A., Chamma É., Kébir H., Gorina R., Lécuyer M.A., Alvarez J.I., De Koninck Y., Engelhardt B., et al. . 2014. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J. Immunol. 193:2438–2454. 10.4049/jimmunol.1400401 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

