Platelet gene expression and function in patients with COVID-19
- PMID: 32573711
- PMCID: PMC7483430
- DOI: 10.1182/blood.2020007214
Platelet gene expression and function in patients with COVID-19
Abstract
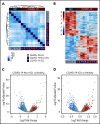
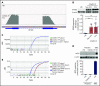
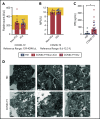
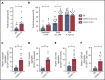
There is an urgent need to understand the pathogenesis of coronavirus disease 2019 (COVID-19). In particular, thrombotic complications in patients with COVID-19 are common and contribute to organ failure and mortality. Patients with severe COVID-19 present with hemostatic abnormalities that mimic disseminated intravascular coagulopathy associated with sepsis, with the major difference being increased risk of thrombosis rather than bleeding. However, whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection alters platelet function to contribute to the pathophysiology of COVID-19 remains unknown. In this study, we report altered platelet gene expression and functional responses in patients infected with SARS-CoV-2. RNA sequencing demonstrated distinct changes in the gene-expression profile of circulating platelets of COVID-19 patients. Pathway analysis revealed differential gene-expression changes in pathways associated with protein ubiquitination, antigen presentation, and mitochondrial dysfunction. The receptor for SARS-CoV-2 binding, angiotensin-converting enzyme 2 (ACE2), was not detected by messenger RNA (mRNA) or protein in platelets. Surprisingly, mRNA from the SARS-CoV-2 N1 gene was detected in platelets from 2 of 25 COVID-19 patients, suggesting that platelets may take-up SARS-COV-2 mRNA independent of ACE2. Resting platelets from COVID-19 patients had increased P-selectin expression basally and upon activation. Circulating platelet-neutrophil, -monocyte, and -T-cell aggregates were all significantly elevated in COVID-19 patients compared with healthy donors. Furthermore, platelets from COVID-19 patients aggregated faster and showed increased spreading on both fibrinogen and collagen. The increase in platelet activation and aggregation could partially be attributed to increased MAPK pathway activation and thromboxane generation. These findings demonstrate that SARS-CoV-2 infection is associated with platelet hyperreactivity, which may contribute to COVID-19 pathophysiology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Comment in
-
COVID-19 concerns aggregate around platelets.Blood. 2020 Sep 10;136(11):1221-1223. doi: 10.1182/blood.2020007805. Blood. 2020. PMID: 32957116 Free PMC article.
-
ART and science of keeping HIV out of the blood supply.Blood. 2020 Sep 10;136(11):1223-1224. doi: 10.1182/blood.2020008122. Blood. 2020. PMID: 32957118 No abstract available.
-
Platelets are Hyperactivated but Show Reduced Glycoprotein VI Reactivity in COVID-19 Patients.Thromb Haemost. 2021 Sep;121(9):1258-1262. doi: 10.1055/a-1347-5555. Epub 2021 Apr 14. Thromb Haemost. 2021. PMID: 33412595 No abstract available.
References
-
- Poissy J, Goutay J, Caplan M, et al. ; Lille ICU Haemostasis COVID-19 group . Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184-186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 HL135265/HL/NHLBI NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- S10 RR026802/RR/NCRR NIH HHS/United States
- R01 HL130541/HL/NHLBI NIH HHS/United States
- R01 HD093826/HD/NICHD NIH HHS/United States
- R01 HL142804/HL/NHLBI NIH HHS/United States
- I01 CX001696/CX/CSRD VA/United States
- R00 HL135265/HL/NHLBI NIH HHS/United States
- R35 HL145237/HL/NHLBI NIH HHS/United States
- R56 AG059877/AG/NIA NIH HHS/United States
- K01 AG059892/AG/NIA NIH HHS/United States
- R01 AG048022/AG/NIA NIH HHS/United States
- R01 HL144957/HL/NHLBI NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

