Well-Defined, Molecular Bismuth Compounds: Catalysts in Photochemically Induced Radical Dehydrocoupling Reactions
- PMID: 32573876
- PMCID: PMC7821184
- DOI: 10.1002/chem.202002219
Well-Defined, Molecular Bismuth Compounds: Catalysts in Photochemically Induced Radical Dehydrocoupling Reactions
Abstract
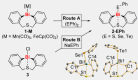
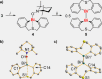
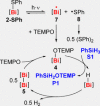
A series of diorgano(bismuth)chalcogenides, [Bi(di-aryl)EPh], has been synthesised and fully characterised (E=S, Se, Te). These molecular bismuth complexes have been exploited in homogeneous photochemically-induced radical catalysis, using the coupling of silanes with TEMPO as a model reaction (TEMPO=(tetramethyl-piperidin-1-yl)-oxyl). Their catalytic properties are complementary or superior to those of known catalysts for these coupling reactions. Catalytically competent intermediates of the reaction have been identified. Applied analytical techniques include NMR, UV/Vis, and EPR spectroscopy, mass spectrometry, single-crystal X-ray diffraction analysis, and (TD)-DFT calculations.
Keywords: bismuth; chalcogens; dehydrocoupling; photocatalysis; radical reactions.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Two Faces of the Bi-O Bond: Photochemically and Thermally Induced Dehydrocoupling for Si-O Bond Formation.Eur J Inorg Chem. 2022 Mar 9;2022(7):e202100934. doi: 10.1002/ejic.202100934. Epub 2021 Dec 18. Eur J Inorg Chem. 2022. PMID: 35873275 Free PMC article.
-
Bismuth Amides Mediate Facile and Highly Selective Pn-Pn Radical-Coupling Reactions (Pn=N, P, As).Angew Chem Int Ed Engl. 2021 Mar 15;60(12):6441-6445. doi: 10.1002/anie.202015514. Epub 2021 Jan 28. Angew Chem Int Ed Engl. 2021. PMID: 33315293 Free PMC article.
-
Bismuth Bound to Transition Metals: Visible-Light-Induced Olefin Coordination, Reductive Elimination, and Oxidative Addition.Chemistry. 2025 Jan 14;31(3):e202403253. doi: 10.1002/chem.202403253. Epub 2024 Nov 21. Chemistry. 2025. PMID: 39498538
-
Molecular Bismuth Cations: Assessment of Soft Lewis Acidity.Chemistry. 2020 Aug 12;26(45):10250-10258. doi: 10.1002/chem.202001674. Epub 2020 Jul 28. Chemistry. 2020. PMID: 32428329 Free PMC article.
-
Organosulphur and organoselenium compounds as emerging building blocks for catalytic systems for O-arylation of phenols, a C-O coupling reaction.Dalton Trans. 2022 May 31;51(21):8103-8132. doi: 10.1039/d1dt04371d. Dalton Trans. 2022. PMID: 35535745 Review.
Cited by
-
Cationic Bismuth Aminotroponiminates: Charge Controls Redox Properties.Chemistry. 2021 Apr 7;27(20):6230-6239. doi: 10.1002/chem.202005186. Epub 2021 Jan 26. Chemistry. 2021. PMID: 33326650 Free PMC article.
-
Catalytic Hydrodefluorination via Oxidative Addition, Ligand Metathesis, and Reductive Elimination at Bi(I)/Bi(III) Centers.J Am Chem Soc. 2021 Aug 18;143(32):12487-12493. doi: 10.1021/jacs.1c06735. Epub 2021 Aug 6. J Am Chem Soc. 2021. PMID: 34358426 Free PMC article.
-
Main Group Redox Catalysis of Organopnictogens: Vertical Periodic Trends and Emerging Opportunities in Group 15.J Am Chem Soc. 2021 Feb 3;143(4):1699-1721. doi: 10.1021/jacs.0c12816. Epub 2021 Jan 19. J Am Chem Soc. 2021. PMID: 33464903 Free PMC article.
-
Isolation and Characterization of an Organobismuth Dihydride.J Am Chem Soc. 2025 Aug 20;147(33):29636-29641. doi: 10.1021/jacs.5c09023. Epub 2025 Aug 5. J Am Chem Soc. 2025. PMID: 40764256 Free PMC article.
-
Bismuth Redox Catalysis: An Emerging Main-Group Platform for Organic Synthesis.ACS Catal. 2022 Jan 21;12(2):1382-1393. doi: 10.1021/acscatal.1c04897. Epub 2022 Jan 7. ACS Catal. 2022. PMID: 35096470 Free PMC article. Review.
References
-
- None
-
- “Bond Dissociation Energies”: Luo Y.-R. in CRC Handbook of Chemistry and Physics, 89th ed. (Ed.: Lide D. R.), CRC/Taylor and Francis, Boca Raton, 2009;
-
- Price S. J. W., Trotman-Dickenson A. F., Trans. Faraday Soc. 1958, 54, 1630–1637;
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

