High molecular weight kininogen contributes to early mortality and kidney dysfunction in a mouse model of sickle cell disease
- PMID: 32573897
- PMCID: PMC8043232
- DOI: 10.1111/jth.14972
High molecular weight kininogen contributes to early mortality and kidney dysfunction in a mouse model of sickle cell disease
Abstract
Background: Sickle cell disease (SCD) is characterized by chronic hemolytic anemia, vaso-occlusive crises, chronic inflammation, and activation of coagulation. The clinical complications such as painful crisis, stroke, pulmonary hypertension, nephropathy and venous thromboembolism lead to cumulative organ damage and premature death. High molecular weight kininogen (HK) is a central cofactor for the kallikrein-kinin and intrinsic coagulation pathways, which contributes to both coagulation and inflammation.
Objective: We hypothesize that HK contributes to the hypercoagulable and pro-inflammatory state that causes end-organ damage and early mortality in sickle mice.
Methods: We evaluated the role of HK in the Townes mouse model of SCD.
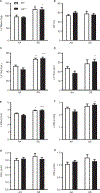
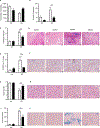
Results/conclusions: We found elevated plasma levels of cleaved HK in sickle patients compared to healthy controls, suggesting ongoing HK activation in SCD. We used bone marrow transplantation to generate wild type and sickle cell mice on a HK-deficient background. We found that short-term HK deficiency attenuated thrombin generation and inflammation in sickle mice at steady state, which was independent of bradykinin signaling. Moreover, long-term HK deficiency attenuates kidney injury, reduces chronic inflammation, and ultimately improves survival of sickle mice.
Keywords: anemia; blood coagulation; high molecular weight; inflammation; kidney disease; kininogen; sickle cell.
© 2020 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
Figures
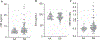
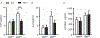


Similar articles
-
Role of the coagulation system in the pathogenesis of sickle cell disease.Blood Adv. 2019 Oct 22;3(20):3170-3180. doi: 10.1182/bloodadvances.2019000193. Blood Adv. 2019. PMID: 31648337 Free PMC article.
-
Olinciguat, a stimulator of soluble guanylyl cyclase, attenuates inflammation, vaso-occlusion and nephropathy in mouse models of sickle cell disease.Br J Pharmacol. 2021 Sep;178(17):3463-3475. doi: 10.1111/bph.15492. Epub 2021 May 30. Br J Pharmacol. 2021. PMID: 33864386 Free PMC article.
-
The Plasma Kallikrein-Kininogen Pathway Is Critical in the Pathogenesis of Colitis in Mice.Front Immunol. 2018 Feb 6;9:21. doi: 10.3389/fimmu.2018.00021. eCollection 2018. Front Immunol. 2018. PMID: 29467753 Free PMC article.
-
Two faces of high-molecular-weight kininogen (HK) in angiogenesis: bradykinin turns it on and cleaved HK (HKa) turns it off.J Thromb Haemost. 2005 Apr;3(4):670-6. doi: 10.1111/j.1538-7836.2005.01218.x. Epub 2005 Feb 23. J Thromb Haemost. 2005. PMID: 15733059 Review.
-
Pharmacological interventions for painful sickle cell vaso-occlusive crises in adults.Cochrane Database Syst Rev. 2019 Nov 14;2019(11):CD012187. doi: 10.1002/14651858.CD012187.pub2. Cochrane Database Syst Rev. 2019. PMID: 31742673 Free PMC article.
Cited by
-
Blocking domain 6 of high molecular weight kininogen to understand intrinsic clotting mechanisms.Res Pract Thromb Haemost. 2022 Oct 13;6(7):e12815. doi: 10.1002/rth2.12815. eCollection 2022 Oct. Res Pract Thromb Haemost. 2022. PMID: 36254255 Free PMC article.
-
Kininogen enhances seizure susceptibility in mice possibly through bradykinin-induced modulation of calcium transients in glutamatergic and GABAergic neurons.Front Pharmacol. 2025 Jun 10;16:1509837. doi: 10.3389/fphar.2025.1509837. eCollection 2025. Front Pharmacol. 2025. PMID: 40556759 Free PMC article.
-
The contact activation system and vascular factors as alternative targets for Alzheimer's disease therapy.Res Pract Thromb Haemost. 2021 May 3;5(4):e12504. doi: 10.1002/rth2.12504. eCollection 2021 May. Res Pract Thromb Haemost. 2021. PMID: 33977208 Free PMC article.
-
Anti-HK antibody inhibits the plasma contact system by blocking prekallikrein and factor XI activation in vivo.Blood Adv. 2023 Apr 11;7(7):1156-1167. doi: 10.1182/bloodadvances.2021006485. Blood Adv. 2023. PMID: 36409609 Free PMC article.
-
Factor XII contributes to thrombotic complications and vaso-occlusion in sickle cell disease.Blood. 2023 Apr 13;141(15):1871-1883. doi: 10.1182/blood.2022017074. Blood. 2023. PMID: 36706361 Free PMC article.
References
-
- Setty BN, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost 2008;6(12):2202–2209. - PubMed
-
- Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program 2007;2007(1):91–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 16POST30230002/American Heart Association/International
- R01 NS102721/NS/NINDS NIH HHS/United States
- U01 HL117659/HL/NHLBI NIH HHS/United States
- T32 HL007149/HL/NHLBI NIH HHS/United States
- R01 HL157441/HL/NHLBI NIH HHS/United States
- U01 HL117684/HL/NHLBI NIH HHS/United States
- K99 HL144817/HL/NHLBI NIH HHS/United States
- R00 HL144817/HL/NHLBI NIH HHS/United States
- R01 HL142604/HL/NHLBI NIH HHS/United States
- R01 HL146226/HL/NHLBI NIH HHS/United States
- R01NS102721/NS/NINDS NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

