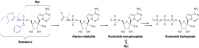Available Evidence and Ongoing Clinical Trials of Remdesivir: Could It Be a Promising Therapeutic Option for COVID-19?
- PMID: 32574236
- PMCID: PMC7264155
- DOI: 10.3389/fphar.2020.00791
Available Evidence and Ongoing Clinical Trials of Remdesivir: Could It Be a Promising Therapeutic Option for COVID-19?
Abstract
The novel coronavirus strain, severe acute respiratory syndrome coronavirus-2, the causative agent of COVID-19 emerged in Wuhan, China, in December 2019 and is skyrocketing throughout the globe and become a global public health emergency. Despite promising preventive measures being taken, there is no vaccine or drug therapy officially approved to prevent or treat the infection. Everybody is waiting the findings of ongoing clinical trials in various chemical and biological products. This review is specifically aimed to summarize the available evidence and ongoing clinical trials of remdesivir as a potential therapeutic option for COVID-19. Remdesivir is an investigational drug having broad spectrum antiviral activity with its target RNA dependent RNA polymerase. It has not yet been officially approved for Ebola and Coronaviruses. Several studies showed that remdesivir had promising in vitro and in vivo antiviral activities against SARS-CoV-1 and MERS-CoV strains. On the top of this, it exhibited a promising in vitro activity against SARS-CoV-2 strains though there are no published studies that substantiate its activity in vivo until the time of this review. There are few phase 3 randomized double-blind placebo controlled trials on the way to investigate the safety and efficacy of remdesivir. Of which, one completed double blind, placebo controlled trial showed that remdesivir showed faster time to clinical improvement in severe COVID-19 patients compared to placebo though not found statistically significant. In addition, two phase 3 randomized open label clinical trials coordinated by Gilead Sciences are being conducted. In addition, WHO Solidarity trial and INSERM DisCoVeRy trials (randomized open labels) were launched recently.
Keywords: COVID-19; GS-5734; RdRp; SARS-CoV-2; remdesivir.
Copyright © 2020 Sisay.
Figures
References
-
- Branswell H. (2020). WHO to launch multinational trial to jumpstart search for coronavirus drugs (World Health Organization; ). Available at: https://www.statnews.com/2020/03/18/who-to-launch-multinational-trial-to... Accessed on 20 March, 2020.
-
- Brown A. J., Won J. J., Graham R. L., Dinnon III K. H., Sims A. C., Feng J. Y., et al. (2019). Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 169, 104541. 10.1016/j.antiviral.2019.104541 - DOI - PMC - PubMed
-
- Cao B. (2020. a). Mild/Moderate 2019-nCoV Remdesivir RCT, Available at: https://clinicaltrials.gov/ct2/show/NCT04252664 Accessed on 20, March, 2020.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous