Lessons Learned to Date on COVID-19 Hyperinflammatory Syndrome: Considerations for Interventions to Mitigate SARS-CoV-2 Viral Infection and Detrimental Hyperinflammation
- PMID: 32574265
- PMCID: PMC7272717
- DOI: 10.3389/fimmu.2020.01131
Lessons Learned to Date on COVID-19 Hyperinflammatory Syndrome: Considerations for Interventions to Mitigate SARS-CoV-2 Viral Infection and Detrimental Hyperinflammation
Abstract
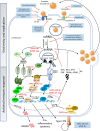
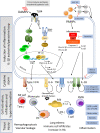
The first case of human transmission of SARS-CoV-2 was reported in China in December 2019. A few months later, this viral infection had spread worldwide and became a pandemic. The disease caused by SARS-CoV-2, termed COVID-19, is multifactorial and associated with both specific antiviral as well as inflammatory responses, the extent of which may determine why some individuals are asymptomatic while others develop serious complications. Here we review possible life-threating immune events that can occur during disease progression to uncover key factors behind COVID-19 severity and provide suggestions for interventions with repurposed drugs in well-controlled and randomized clinical trials. These drugs include therapeutics with potential to inhibit SARS-CoV-2 entry into host cells such as serine protease inhibitors of the cellular protease TMPS2 and drugs targeting the renin-angiotensin system; antivirals with potential to block SARS-CoV-2 replication or factors that could boost the antiviral response; monoclonal antibodies targeting pro-inflammatory cytokines that drive the hyperinflammatory response during COVID-19 progression toward the severe stage and therapeutics that could ameliorate the function of the lungs. Furthermore, in order to help make more informed decisions on the timing of the intervention with the drugs listed in this review, we have grouped these therapeutics according to the stage of COVID-19 progression that we considered most appropriate for their mechanism of action.
Keywords: 2019-nCoV; SARS-CoV-2; antiviral immune response; coronavirus; cytokine release syndrome; hyperinflammation; severe COVID-19; treatment strategies.
Copyright © 2020 Cardone, Yano, Rosenberg and Puig.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

