Symptomatic Protective Action of Glycyrrhizin (Licorice) in COVID-19 Infection?
- PMID: 32574273
- PMCID: PMC7270278
- DOI: 10.3389/fimmu.2020.01239
Symptomatic Protective Action of Glycyrrhizin (Licorice) in COVID-19 Infection?
Abstract
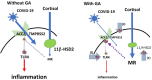
The role of the ACE2 enzyme in the COVID-19 infection is 2-fold, with opposing implications for the disease development. 1. The membrane bound angiotensin converting enzyme 2 (ACE2) serves as the entry point of COVID-19 2. Conversely, it supports an anti-inflammatory pathway. This led to the controversy of the impact of medications, which influence its expression. ACE2 is part of the wider renin-angiotensin-aldosterone system (RAAS) and is upregulated via compounds, which inhibits the classical ACE, thereby plasma aldosterone and aldosterone receptor (MR) activation. MR activation may therefore protect organs from binding the COVID-19 by reducing ACE2 expression. Glycyrrhizin (GL) is a frequent component in traditional Chinese medicines, which have been used to control COVID-19 infections. Its systemically active metabolite glycyrrhetinic acid (GA) inhibits 11beta hydroxysteroid dehydrogenase(11betaHSD2) and activates MR in organs, which express this enzyme, including the lungs. Does this affect the protective effect of ACE2? Importantly, GL has anti-inflammatory properties by itself via toll like receptor 4 (TLR4) antagonism and therefore compensates for the reduced protection of the downregulated ACE2. Finally, a direct effect of GL or GA to reduce virus transmission exists, which may involve reduced expression of type 2 transmembrane serine protease (TMPRSS2), which is required for virus uptake. Glycyrrhizin may reduce the severity of an infection with COVID-19 at the two stages of the COVID-19 induced disease process, 1. To block the number of entry points and 2. provide an ACE2 independent anti-inflammatory mechanism.
Keywords: 11 beta hydroxysteroid dehydrogenase; COVID-19; Corona virus; angiotensin converrting enzyme; glycyrrhizin; inflammation; mineralocorticoid receptor; toll like receptor 4 (TLR4).
Copyright © 2020 Murck.
Figures
Similar articles
-
Food Enrichment with Glycyrrhiza glabra Extract Suppresses ACE2 mRNA and Protein Expression in Rats-Possible Implications for COVID-19.Nutrients. 2021 Jul 6;13(7):2321. doi: 10.3390/nu13072321. Nutrients. 2021. PMID: 34371831 Free PMC article.
-
Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19.Int J Antimicrob Agents. 2020 Jun;55(6):105995. doi: 10.1016/j.ijantimicag.2020.105995. Epub 2020 Apr 24. Int J Antimicrob Agents. 2020. PMID: 32335281 Free PMC article.
-
Can Antiviral Activity of Licorice Help Fight COVID-19 Infection?Biomolecules. 2021 Jun 8;11(6):855. doi: 10.3390/biom11060855. Biomolecules. 2021. PMID: 34201172 Free PMC article. Review.
-
Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.J Med Virol. 2020 Jul;92(7):726-730. doi: 10.1002/jmv.25785. Epub 2020 Apr 5. J Med Virol. 2020. PMID: 32221983 Free PMC article. Review.
-
Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers.Hypertension. 2020 Jun;75(6):1382-1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. Epub 2020 Mar 25. Hypertension. 2020. PMID: 32208987 Free PMC article.
Cited by
-
A Novel Handrub Tablet Loaded with Pre- and Post-Biotic Solid Lipid Nanoparticles Combining Virucidal Activity and Maintenance of the Skin Barrier and Microbiome.Pharmaceutics. 2023 Dec 17;15(12):2793. doi: 10.3390/pharmaceutics15122793. Pharmaceutics. 2023. PMID: 38140133 Free PMC article.
-
Medicinal Plants, Phytochemicals, and Herbs to Combat Viral Pathogens Including SARS-CoV-2.Molecules. 2021 Mar 22;26(6):1775. doi: 10.3390/molecules26061775. Molecules. 2021. PMID: 33809963 Free PMC article. Review.
-
Understanding COVID-19 in Wuhan From the Perspective of Cold-Dampness: Clinical Evidences and Mechanisms.Front Med (Lausanne). 2021 Feb 22;8:617659. doi: 10.3389/fmed.2021.617659. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33693014 Free PMC article. Review.
-
Role of ACE2-Ang (1-7)-Mas axis in post-COVID-19 complications and its dietary modulation.Mol Cell Biochem. 2022 Jan;477(1):225-240. doi: 10.1007/s11010-021-04275-2. Epub 2021 Oct 16. Mol Cell Biochem. 2022. PMID: 34655418 Free PMC article. Review.
-
Traditional Uses, Pharmacological Effects, and Molecular Mechanisms of Licorice in Potential Therapy of COVID-19.Front Pharmacol. 2021 Nov 26;12:719758. doi: 10.3389/fphar.2021.719758. eCollection 2021. Front Pharmacol. 2021. PMID: 34899289 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

