New Insights of Emerging SARS-CoV-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and Prevention
- PMID: 32574318
- PMCID: PMC7256189
- DOI: 10.3389/fcell.2020.00410
New Insights of Emerging SARS-CoV-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and Prevention
Abstract
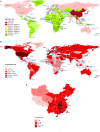
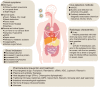
Since the first reports that the novel coronavirus was showing human-to-human transmission characteristics and asymptomatic cases, the number of patients with associated pneumonia has continued to rise and the epidemic has grown. It now threatens the health and lives of people across the world. The governments of many countries have attached great importance to the prevention of SARS-CoV-2, via research into the etiology and epidemiology of this newly emerged disease. Clinical signs, treatment, and prevention characteristics of the novel coronavirus pneumonia have been receiving attention worldwide, especially from medical personnel. However, owing to the different experimental methods, sample sizes, sample sources, and research perspectives of various studies, results have been inconsistent, or relate to an isolated aspect of the virus or the disease it causes. Currently, systematic summary data on the novel coronavirus are limited. This review combines experimental and clinical evidence into a systematic analysis and summary of the current progress of research into SARS-CoV-2, from multiple perspectives, with the aim of gaining a better overall understanding of the disease. Our report provides important information for current clinicians, for the prevention and treatment of COVID-19 pneumonia.
Keywords: SARS-CoV-2; clinical features; clinical treatment and prevention; coronavirus; epidemiology; etiology.
Copyright © 2020 Guo, Ye, Pan, Chen, Xing, Yan, Chen, Ding, Li, Huang, Zhang, Li and Xue.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

