Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context
- PMID: 32574326
- PMCID: PMC7227790
- DOI: 10.3389/fmed.2020.00225
Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context
Abstract
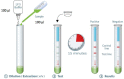
Introduction: COVID-19 Ag Respi-Strip, an immunochromatographic (ICT) assay for the rapid detection of SARS-CoV-2 antigen on nasopharyngeal specimen, has been developed to identify positive COVID-19 patients allowing prompt clinical and quarantine decisions. In this original research article, we describe the conception, the analytical and clinical performances as well as the risk management of implementing the COVID-19 Ag Respi-Strip in a diagnostic decision algorithm. Materials and Methods: Development of the COVID-19 Ag Respi-Strip resulted in a ready-to-use ICT assay based on a membrane technology with colloidal gold nanoparticles using monoclonal antibodies directed against the SARS-CoV and SARS-CoV-2 highly conserved nucleoprotein antigen. Four hundred observations were recorded for the analytical performance study and thirty tests were analyzed for the cross-reactivity study. The clinical performance study was performed in a retrospective multi-centric evaluation on aliquots of 328 nasopharyngeal samples. COVID-19 Ag Respi-Strip results were compared with qRT-PCR as golden standard for COVID-19 diagnostics. Results: In the analytical performance study, the reproducibility showed a between-observer disagreement of 1.7%, a robustness of 98%, an overall satisfying user friendliness and no cross-reactivity with other virus-infected nasopharyngeal samples. In the clinical performance study performed in three different clinical laboratories during the ascendant phase of the epidemiological curve, we found an overall sensitivity and specificity of 57.6 and 99.5%, respectively with an accuracy of 82.6%. The cut-off of the ICT was found at CT <22. User-friendliness analysis and risk management assessment through Ishikawa diagram demonstrate that COVID-19 Ag Respi-Strip may be implemented in clinical laboratories according to biosafety recommendations. Conclusion: The COVID-19 Ag Respi-Strip represents a promising rapid SARS-CoV-2 antigen assay for the first-line diagnosis of COVID-19 in 15 min at the peak of the pandemic. Its role in the proposed diagnostic algorithm is complementary to the currently-used molecular techniques.
Keywords: COVID-19; SARS-CoV-2; antigen; diagnostic; immunochromatographic test.
Copyright © 2020 Mertens, De Vos, Martiny, Jassoy, Mirazimi, Cuypers, Van den Wijngaert, Monteil, Melin, Stoffels, Yin, Mileto, Delaunoy, Magein, Lagrou, Bouzet, Serrano, Wautier, Leclipteux, Van Ranst, Vandenberg and LHUB-ULB SARS-CoV-2 working diagnostic group.
Figures


Comment in
-
Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: Experiences of a general hospital.J Clin Virol. 2020 Aug;129:104472. doi: 10.1016/j.jcv.2020.104472. Epub 2020 May 30. J Clin Virol. 2020. PMID: 32504944 Free PMC article.
References
-
- Che XY, Qiu LW, Pan YX, Wen K, Hao W, Zhang LY, et al. . Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J Clin Microbiol. (2004) 42:2629–35. 10.1128/JCM.42.6.2629-2635.2004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

