Antifibrotic Drug Use in Patients with Idiopathic Pulmonary Fibrosis. Data from the IPF-PRO Registry
- PMID: 32574517
- PMCID: PMC7640723
- DOI: 10.1513/AnnalsATS.201912-880OC
Antifibrotic Drug Use in Patients with Idiopathic Pulmonary Fibrosis. Data from the IPF-PRO Registry
Abstract
Rationale: Two antifibrotic medications, nintedanib and pirfenidone, have been approved for the treatment of idiopathic pulmonary fibrosis (IPF) in the United States. Few data have been published on the use of these medications in clinical practice.Objectives: To investigate patterns of use of antifibrotic medications in the United States.Methods: The Idiopathic Pulmonary Fibrosis Prospective Outcomes (IPF-PRO) Registry, a multicenter U.S. registry, has enrolled patients with IPF that was diagnosed or confirmed at the enrolling center in the past 6 months. Data from patients enrolled from June 5, 2014, to March 4, 2018, were used to determine antifibrotic medication use ("treatment") in the enrollment window and in a follow-up window approximately 6 months later. Associations between patient characteristics and treatment status were tested using logistic regression.Results: Overall, 551 of 782 eligible patients (70.5%) were treated in the enrollment window. Younger age, lower forced vital capacity percentage predicted, oxygen use with activity, worse self-rated health (based on the Short Form 12 or St. George's Respiratory Questionnaire score), referral to the enrolling center by a pulmonologist, use of a lung biopsy in diagnosis, and carrying a diagnosis of IPF to the enrolling center were associated with being treated. Among 534 patients treated at enrollment who had follow-up data, 94.0% remained treated in follow-up. Better self-rated health (based on the Short Form 12 mental component score or EuroQoL score) and not using oxygen with activity at enrollment were associated with continuing treatment in follow-up. Among 172 patients who were untreated at enrollment and had follow-up data, 29.7% started treatment in follow-up. Lower diffusing capacity of the lung for carbon monoxide percentage predicted, a family history of interstitial lung disease, a history of sleep apnea, and a definite diagnosis of IPF at enrollment were associated with starting treatment in follow-up.Conclusions: The majority of patients in the IPF-PRO Registry were receiving an approved medication for IPF at enrollment. Treatment at enrollment was associated with greater disease severity, more compromised quality of life, and the use of oxygen with activity.Clinical trial registered with ClinicalTrials.gov (NCT01915511).
Keywords: clinical practice patterns; idiopathic pulmonary fibrosis; interstitial lung disease; treatment.
Figures
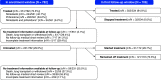

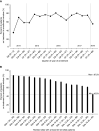

Comment in
-
Registries for Idiopathic Pulmonary Fibrosis: When Is It Time to Go Global?Ann Am Thorac Soc. 2020 Nov;17(11):1378-1379. doi: 10.1513/AnnalsATS.202007-835ED. Ann Am Thorac Soc. 2020. PMID: 33124912 Free PMC article. No abstract available.
References
-
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. - PubMed
-
- Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. - PubMed
-
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. - PubMed
-
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

