IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice With Steatohepatitis
- PMID: 32574624
- PMCID: PMC7666601
- DOI: 10.1053/j.gastro.2020.06.031
IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice With Steatohepatitis
Erratum in
-
Correction.Gastroenterology. 2022 Apr;162(4):1363-1365. doi: 10.1053/j.gastro.2022.01.031. Epub 2022 Feb 3. Gastroenterology. 2022. PMID: 35122740 No abstract available.
Abstract
Background & aims: Endoplasmic reticulum to nucleus signaling 1 (ERN1, also called IRE1A) is a sensor of the unfolded protein response that is activated in the livers of patients with nonalcoholic steatohepatitis (NASH). Hepatocytes release ceramide-enriched inflammatory extracellular vesicles (EVs) after activation of IRE1A. We studied the effects of inhibiting IRE1A on release of inflammatory EVs in mice with diet-induced steatohepatitis.
Methods: C57BL/6J mice and mice with hepatocyte-specific disruption of Ire1a (IRE1αΔhep) were fed a diet high in fat, fructose, and cholesterol to induce development of steatohepatitis or a standard chow diet (controls). Some mice were given intraperitoneal injections of the IRE1A inhibitor 4μ8C. Mouse liver and primary hepatocytes were transduced with adenovirus or adeno-associated virus that expressed IRE1A. Livers were collected from mice and analyzed by quantitative polymerase chain reaction and chromatin immunoprecipitation assays; plasma samples were analyzed by enzyme-linked immunosorbent assay. EVs were derived from hepatocytes and injected intravenously into mice. Plasma EVs were characterized by nanoparticle-tracking analysis, electron microscopy, immunoblots, and nanoscale flow cytometry; we used a membrane-tagged reporter mouse to detect hepatocyte-derived EVs. Plasma and liver tissues from patients with NASH and without NASH (controls) were analyzed for EV concentration and by RNAscope and gene expression analyses.
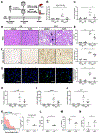
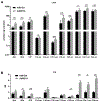
Results: Disruption of Ire1a in hepatocytes or inhibition of IRE1A reduced the release of EVs and liver injury, inflammation, and accumulation of macrophages in mice on the diet high in fat, fructose, and cholesterol. Activation of IRE1A, in the livers of mice, stimulated release of hepatocyte-derived EVs, and also from cultured primary hepatocytes. Mice given intravenous injections of IRE1A-stimulated, hepatocyte-derived EVs accumulated monocyte-derived macrophages in the liver. IRE1A-stimulated EVs were enriched in ceramides. Chromatin immunoprecipitation showed that IRE1A activated X-box binding protein 1 (XBP1) to increase transcription of serine palmitoyltransferase genes, which encode the rate-limiting enzyme for ceramide biosynthesis. Administration of a pharmacologic inhibitor of serine palmitoyltransferase to mice reduced the release of EVs. Levels of XBP1 and serine palmitoyltransferase were increased in liver tissues, and numbers of EVs were increased in plasma, from patients with NASH compared with control samples and correlated with the histologic features of inflammation.
Conclusions: In mouse hepatocytes, activated IRE1A promotes transcription of serine palmitoyltransferase genes via XBP1, resulting in ceramide biosynthesis and release of EVs. The EVs recruit monocyte-derived macrophages to the liver, resulting in inflammation and injury in mice with diet-induced steatohepatitis. Levels of XBP1, serine palmitoyltransferase, and EVs are all increased in liver tissues from patients with NASH. Strategies to block this pathway might be developed to reduce liver inflammation in patients with NASH.
Keywords: ER Stress; Exosome; Lipotoxicity; Macrophage.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Lassailly G, Caiazzo R, Buob D, et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015;149:379–88; quiz e15-6. - PubMed
-
- Gehrke N, Schattenberg JM. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020. - PubMed
-
- Fun XH, Thibault G. Lipid bilayer stress and proteotoxic stress-induced unfolded protein response deploy divergent transcriptional and non-transcriptional programmes. Biochim Biophys Acta Mol Cell Biol Lipids 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

