Compassionate Use of Tocilizumab for Treatment of SARS-CoV-2 Pneumonia
- PMID: 32575124
- PMCID: PMC7337689
- DOI: 10.1093/cid/ciaa812
Compassionate Use of Tocilizumab for Treatment of SARS-CoV-2 Pneumonia
Abstract
Background: Preliminary data from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia patients indicate that a cytokine storm may increase morbidity and mortality. Tocilizumab (anti-IL-6R) is approved by the Food and Drug Administration for treatment of cytokine storm associated with chimeric antigen receptor T-cell therapy. Here we examined compassionate use of tocilizumab in patients with SARS-CoV-2 pneumonia.
Methods: We report on a single-center study of tocilizumab in hospitalized patients with SARS-CoV-2 pneumonia. All patients had confirmed SARS-CoV-2 pneumonia and oxygen saturations <90% on oxygen support with most intubated. We examined clinical and laboratory parameters including oxygen and vasopressor requirements, cytokine profiles, and C-reactive protein (CRP) levels pre- and post-tocilizumab treatment.
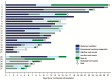
Results: Twenty-seven SARS-CoV-2 pneumonia patients received one 400 mg dose of tocilizumab. Interleukin (IL)-6 was the predominant cytokine detected at tocilizumab treatment. Significant reductions in temperature and CRP were seen post-tocilizumab. However, 4 patients did not show rapid CRP declines, of whom 3 had poorer outcomes. Oxygen and vasopressor requirements diminished over the first week post-tocilizumab. Twenty-two patients required mechanical ventilation; at last follow-up, 16 were extubated. Adverse events and serious adverse events were minimal, but 2 deaths (7.4%) occurred that were felt unrelated to tocilizumab.
Conclusions: Compared to published reports on the morbidity and mortality associated with SARS-CoV-2, tocilizumab appears to offer benefits in reducing inflammation, oxygen requirements, vasopressor support, and mortality. The rationale for tocilizumab treatment is supported by detection of IL-6 in pathogenic levels in all patients. Additional doses of tocilizumab may be needed for those showing slow declines in CRP. Proof of efficacy awaits randomized, placebo-controlled clinical trials.
Keywords: COVID-19; SARS-CoV2; acute respiratory distress syndrome; interleukin 6.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Reply to Olivera and Mallat.Clin Infect Dis. 2021 Jul 1;73(1):e272-e273. doi: 10.1093/cid/ciaa1287. Clin Infect Dis. 2021. PMID: 32886742 No abstract available.
-
Efficacy of Tocilizumab For Treatment of Severe COVID-19 Pneumonia: More Evidence Is Needed.Clin Infect Dis. 2021 Jul 1;73(1):e271-e272. doi: 10.1093/cid/ciaa1284. Clin Infect Dis. 2021. PMID: 32886762 Free PMC article. No abstract available.
References
-
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377:562–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

