An Electronic Health Record Text Mining Tool to Collect Real-World Drug Treatment Outcomes: A Validation Study in Patients With Metastatic Renal Cell Carcinoma
- PMID: 32575147
- PMCID: PMC7484987
- DOI: 10.1002/cpt.1966
An Electronic Health Record Text Mining Tool to Collect Real-World Drug Treatment Outcomes: A Validation Study in Patients With Metastatic Renal Cell Carcinoma
Abstract
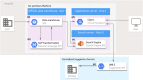
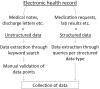
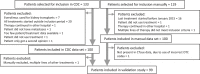
Real-world evidence can close the inferential gap between marketing authorization studies and clinical practice. However, the current standard for real-world data extraction from electronic health records (EHRs) for treatment evaluation is manual review (MR), which is time-consuming and laborious. Clinical Data Collector (CDC) is a novel natural language processing and text mining software tool for both structured and unstructured EHR data and only shows relevant EHR sections improving efficiency. We investigated CDC as a real-world data (RWD) collection method, through application of CDC queries for patient inclusion and information extraction on a cohort of patients with metastatic renal cell carcinoma (RCC) receiving systemic drug treatment. Baseline patient characteristics, disease characteristics, and treatment outcomes were extracted and these were compared with MR for validation. One hundred patients receiving 175 treatments were included using CDC, which corresponded to 99% with MR. Calculated median overall survival was 21.7 months (95% confidence interval (CI) 18.7-24.8) vs. 21.7 months (95% CI 18.6-24.8) and progression-free survival 8.9 months (95% CI 5.4-12.4) vs. 7.6 months (95% CI 5.7-9.4) for CDC vs. MR, respectively. Highest F1-score was found for cancer-related variables (88.1-100), followed by comorbidities (71.5-90.4) and adverse drug events (53.3-74.5), with most diverse scores on international metastatic RCC database criteria (51.4-100). Mean data collection time was 12 minutes (CDC) vs. 86 minutes (MR). In conclusion, CDC is a promising tool for retrieving RWD from EHRs because the correct patient population can be identified as well as relevant outcome data, such as overall survival and progression-free survival.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
-
- Franklin, J.M. & Schneeweiss, S. When and how can real world data analyses substitute for randomized controlled trials? Clin. Pharmacol. Ther. 102, 924–933 (2017). - PubMed
-
- Bothwell, L.E. & Podolsky, S.H. The emergence of the randomized controlled trial. N. Engl. J. Med. 375, 501–504 (2016). - PubMed
-
- Verweij, J. et al Innovation in oncology clinical trial design. Cancer Treat. Rev. 74, 15–20 (2019). - PubMed
-
- Lakdawalla, D.N. et al Predicting real‐world effectiveness of cancer therapies using overall survival and progression‐free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health 20, 866–875 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

