In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress
- PMID: 32575493
- PMCID: PMC7350375
- DOI: 10.3390/pathogens9060489
In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress
Abstract
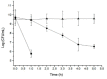
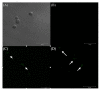
Yeasts can adapt to a wide range of pH fluctuations (2 to 10), while Helicobacter pylori, a facultative intracellular bacterium, can adapt to a range from pH 6 to 8. This work analyzed if H. pylori J99 can protect itself from acidic pH by entering into Candida albicans ATCC 90028. Growth curves were determined for H. pylori and C. albicans at pH 3, 4, and 7. Both microorganisms were co-incubated at the same pH values, and the presence of intra-yeast bacteria was evaluated. Intra-yeast bacteria-like bodies were detected using wet mounting, and intra-yeast binding of anti-H. pylori antibodies was detected using immunofluorescence. The presence of the H. pylori rDNA 16S gene in total DNA from yeasts was demonstrated after PCR amplification. H. pylori showed larger death percentages at pH 3 and 4 than at pH 7. On the contrary, the viability of the yeast was not affected by any of the pHs evaluated. H. pylori entered into C. albicans at all the pH values assayed but to a greater extent at unfavorable pH values (pH 3 or 4, p = 0.014 and p = 0.001, respectively). In conclusion, it is possible to suggest that H. pylori can shelter itself within C. albicans under unfavorable pH conditions.
Keywords: Candida albicans; Helicobacter pylori; intracellular bacteria; pH; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women.Microorganisms. 2021 Jan 8;9(1):131. doi: 10.3390/microorganisms9010131. Microorganisms. 2021. PMID: 33430099 Free PMC article.
-
Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells.Biology (Basel). 2021 Sep 15;10(9):915. doi: 10.3390/biology10090915. Biology (Basel). 2021. PMID: 34571792 Free PMC article.
-
Antibiotics as a Stressing Factor Triggering the Harboring of Helicobacter pylori J99 within Candida albicans ATCC10231.Pathogens. 2021 Mar 23;10(3):382. doi: 10.3390/pathogens10030382. Pathogens. 2021. PMID: 33806815 Free PMC article.
-
Candida albicans Release Intracellular Bacteria When Treated With Amphotericin B.Arch Iran Med. 2018 May 1;21(5):191-198. Arch Iran Med. 2018. PMID: 29738262
-
An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells.Biology (Basel). 2022 May 12;11(5):738. doi: 10.3390/biology11050738. Biology (Basel). 2022. PMID: 35625466 Free PMC article.
Cited by
-
Helicobacter pylori, Protected from Antibiotics and Stresses Inside Candida albicans Vacuoles, Cause Gastritis in Mice.Int J Mol Sci. 2022 Aug 2;23(15):8568. doi: 10.3390/ijms23158568. Int J Mol Sci. 2022. PMID: 35955701 Free PMC article.
-
The Mycobiome: Cancer Pathogenesis, Diagnosis, and Therapy.Cancers (Basel). 2022 Jun 10;14(12):2875. doi: 10.3390/cancers14122875. Cancers (Basel). 2022. PMID: 35740541 Free PMC article. Review.
-
Intracellular Presence of Helicobacter pylori and Its Virulence-Associated Genotypes within the Vaginal Yeast of Term Pregnant Women.Microorganisms. 2021 Jan 8;9(1):131. doi: 10.3390/microorganisms9010131. Microorganisms. 2021. PMID: 33430099 Free PMC article.
-
Surface adherence and vacuolar internalization of bacterial pathogens to the Candida spp. cells: Mechanism of persistence and propagation.J Adv Res. 2023 Nov;53:115-136. doi: 10.1016/j.jare.2022.12.013. Epub 2022 Dec 23. J Adv Res. 2023. PMID: 36572338 Free PMC article. Review.
-
Intracellular presence of Helicobacter pylori antigen and genes within gastric and vaginal Candida.PLoS One. 2024 Feb 8;19(2):e0298442. doi: 10.1371/journal.pone.0298442. eCollection 2024. PLoS One. 2024. PMID: 38329956 Free PMC article.
References
-
- McFall-Ngai M., Hadfield M.G., Bosch T.C.G., Carey H.V., Domazet-Lošo T., Douglas A.E., Dubilier N., Eberl G., Fukami T., Gilbert S.F., et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Nat. Acad. Sci. USA. 2013;110:3229–3236. doi: 10.1073/pnas.1218525110. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

