Angiopoietins, Vascular Endothelial Growth Factors and Secretory Phospholipase A2 in Ischemic and Non-Ischemic Heart Failure
- PMID: 32575548
- PMCID: PMC7356305
- DOI: 10.3390/jcm9061928
Angiopoietins, Vascular Endothelial Growth Factors and Secretory Phospholipase A2 in Ischemic and Non-Ischemic Heart Failure
Abstract
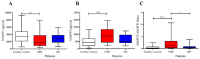
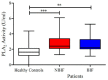
Heart failure (HF) is a growing public health burden, with high prevalence and mortality rates. In contrast to ischemic heart failure (IHF), the diagnosis of non-ischemic heart failure (NIHF) is established in the absence of coronary artery disease. Angiopoietins (ANGPTs), vascular endothelial growth factors (VEGFs) and secretory phospholipases A2 (sPLA2s) are proinflammatory mediators and key regulators of endothelial cells. In the present manuscript, we analyze the plasma concentrations of angiogenic (ANGPT1, ANGPT2, VEGF-A) and lymphangiogenic (VEGF-C, VEGF-D) factors and the plasma activity of sPLA2 in patients with IHF and NIHF compared to healthy controls. The concentrations of ANGPT1, ANGPT2 and their ratio significantly differed between HF patients and healthy controls. Similarly, plasma levels of VEGF-D and sPLA2 activity were higher in HF as compared to controls. Concentrations of ANGPT2 and the ANGPT2/ANGPT1 ratio (an index of vascular permeability) were increased in NIHF patients. VEGF-A and VEGF-C concentrations did not differ among the three examined groups. Interestingly, VEGF-D was selectively increased in IFH patients compared to controls. Plasma activity of sPLA2 was increased in IHF and NIHF patients compared to controls. Our results indicate that several regulators of vascular permeability and smoldering inflammation are specifically altered in IHF and NIHF patients. Studies involving larger cohorts of these patients will be necessary to demonstrate the clinical implications of our findings.
Keywords: IHF; NIHF; VEGFs; angiopoietins; heart failure; sPLA2.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures





References
-
- Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. - PubMed
-
- Bozkurt B., Colvin M., Cook J., Cooper L.T., Deswal A., Fonarow G.C., Francis G.S., Lenihan D., Lewis E.F., McNamara D.M., et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the american heart association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

