Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Pathophysiology and Clinical Implications
- PMID: 32575683
- PMCID: PMC7352421
- DOI: 10.3390/ijms21124391
Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Pathophysiology and Clinical Implications
Abstract
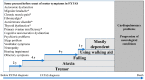
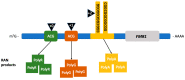
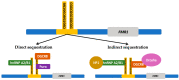
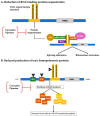
The fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder seen in older premutation (55-200 CGG repeats) carriers of FMR1. The premutation has excessive levels of FMR1 mRNA that lead to toxicity and mitochondrial dysfunction. The clinical features usually begin in the 60 s with an action or intention tremor followed by cerebellar ataxia, although 20% have only ataxia. MRI features include brain atrophy and white matter disease, especially in the middle cerebellar peduncles, periventricular areas, and splenium of the corpus callosum. Neurocognitive problems include memory and executive function deficits, although 50% of males can develop dementia. Females can be less affected by FXTAS because of a second X chromosome that does not carry the premutation. Approximately 40% of males and 16% of female carriers develop FXTAS. Since the premutation can occur in less than 1 in 200 women and 1 in 400 men, the FXTAS diagnosis should be considered in patients that present with tremor, ataxia, parkinsonian symptoms, neuropathy, and psychiatric problems. If a family history of a fragile X mutation is known, then FMR1 DNA testing is essential in patients with these symptoms.
Keywords: FMR1; FMRP; ataxia; fragile X-associated tremor/ataxia syndrome (FXTAS); neurodegeneration; neuroradiology; premutation; tremor.
Conflict of interest statement
R.J.H. has received funding from Zynerba, Ovid, and the Azrieli Foundation for treatment trials in FXS. R.J.H. has also consulted with Zynerba and Fulcrum regarding the organization of clinical trials in FXS. We also acknowledge funding from NICHD (HD036071) and the MIND Institute IDDRC funding from NICHD (U54 HD079125).
Figures

References
-
- Quartier A., Poquet H., Gilbert-Dussardier B., Rossi M., Casteleyn A.S., Des Portes V., Feger C., Nourisson E., Kuentz P., Redin C., et al. Intragenic FMR1 disease-causing variants: A significant mutational mechanism leading to Fragile-X syndrome. Eur. J. Hum. Genet. 2017;25:423–431. doi: 10.1038/ejhg.2016.204. - DOI - PMC - PubMed

