Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/ δ-Deficient Mice
- PMID: 32575793
- PMCID: PMC7353058
- DOI: 10.3390/ijms21124403
Investigating the Role of PPARβ/δ in Retinal Vascular Remodeling Using Pparβ/ δ-Deficient Mice
Abstract
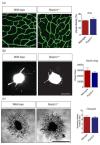
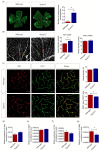
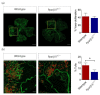
Peroxisome proliferator-activated receptor (PPAR)β/δ is a member of the nuclear receptor superfamily of transcription factors, which plays fundamental roles in cell proliferation and differentiation, inflammation, adipogenesis, and energy homeostasis. Previous studies demonstrated a reduced choroidal neovascularization (CNV) in Pparβ/δ-deficient mice. However, PPARβ/δ's role in physiological blood vessel formation and vessel remodeling in the retina has yet to be established. Our study showed that PPARβ/δ is specifically required for disordered blood vessel formation in the retina. We further demonstrated an increased arteriovenous crossover and wider venous caliber in Pparβ/δ-haplodeficient mice. In summary, these results indicated a critical role of PPARβ/δ in pathological angiogenesis and blood vessel remodeling in the retina.
Keywords: PPARβ/δ; angiogenesis; arteriovenous crossover; blood vessel remodeling; pericytes; vessel caliber.
Conflict of interest statement
S.Y.H., None; Y.P.K., None; B.Y.Q., None; A.T., None; H.M., None; A.V.B., None; N.S.T., None; C.M.C.G., Bayer (C, F), Novartis (C, F), Roche (F), GlaxoSmith Kline (F); T.Y.W., Bayer (C, F), Novartis (C, F), Abbott (C, F), Allergan (C, F), Genentech (C, F), Roche (C, F), Pfizer (C, F); W.W., None; and X.W., None. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

