Multifaceted Roles of Mitochondrial Components and Metabolites in Metabolic Diseases and Cancer
- PMID: 32575796
- PMCID: PMC7352686
- DOI: 10.3390/ijms21124405
Multifaceted Roles of Mitochondrial Components and Metabolites in Metabolic Diseases and Cancer
Abstract
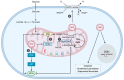
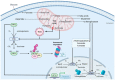
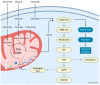
Mitochondria are essential cellular components that ensure physiological metabolic functions. They provide energy in the form of adenosine triphosphate (ATP) through the electron transport chain (ETC). They also constitute a metabolic hub in which metabolites are used and processed, notably through the tricarboxylic acid (TCA) cycle. These newly generated metabolites have the capacity to feed other cellular metabolic pathways; modify cellular functions; and, ultimately, generate specific phenotypes. Mitochondria also provide intracellular signaling cues through reactive oxygen species (ROS) production. As expected with such a central cellular role, mitochondrial dysfunctions have been linked to many different diseases. The origins of some of these diseases could be pinpointed to specific mutations in both mitochondrial- and nuclear-encoded genes. In addition to their impressive intracellular tasks, mitochondria also provide intercellular signaling as they can be exchanged between cells, with resulting effects ranging from repair of damaged cells to strengthened progression and chemo-resistance of cancer cells. Several therapeutic options can now be envisioned to rescue mitochondria-defective cells. They include gene therapy for both mitochondrial and nuclear defective genes. Transferring exogenous mitochondria to target cells is also a whole new area of investigation. Finally, supplementing targeted metabolites, possibly through microbiota transplantation, appears as another therapeutic approach full of promises.
Keywords: cancer; electron transport chain (ETC); metabolism; metabolites; microbiota; mitochondria; mitochondria exchange; mitochondrial DNA (mtDNA); therapy; tricarboxylic acid (TCA) cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

