Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation
- PMID: 32575812
- PMCID: PMC7352415
- DOI: 10.3390/ijms21124407
Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation
Abstract
Extracellular vesicles can cross the blood-brain barrier (BBB), but little is known about passage. Here, we used multiple-time regression analysis to examine the ability of 10 exosome populations derived from mouse, human, cancerous, and non-cancerous cell lines to cross the BBB. All crossed the BBB, but rates varied over 10-fold. Lipopolysaccharide (LPS), an activator of the innate immune system, enhanced uptake independently of BBB disruption for six exosomes and decreased uptake for one. Wheatgerm agglutinin (WGA) modulated transport of five exosome populations, suggesting passage by adsorptive transcytosis. Mannose 6-phosphate inhibited uptake of J774A.1, demonstrating that its BBB transporter is the mannose 6-phosphate receptor. Uptake rates, patterns, and effects of LPS or WGA were not predicted by exosome source (mouse vs. human) or cancer status of the cell lines. The cell surface proteins CD46, AVβ6, AVβ3, and ICAM-1 were variably expressed but not predictive of transport rate nor responses to LPS or WGA. A brain-to-blood efflux mechanism variably affected CNS retention and explains how CNS-derived exosomes enter blood. In summary, all exosomes tested here readily crossed the BBB, but at varying rates and by a variety of vesicular-mediated mechanisms involving specific transporters, adsorptive transcytosis, and a brain-to-blood efflux system.
Keywords: adsorptive transcytosis; blood–brain barrier; diapedesis; exosome; extracellular vesicles; neuroinflammation; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

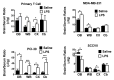
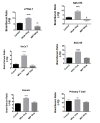

References
-
- Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
-
- Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H., Mark M.T., Molina H., Martin A.B., Bojmar L., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

