A New Selective PPARγ Modulator Inhibits Triglycerides Accumulation during Murine Adipocytes' and Human Adipose-Derived Mesenchymal Stem Cells Differentiation
- PMID: 32575918
- PMCID: PMC7352648
- DOI: 10.3390/ijms21124415
A New Selective PPARγ Modulator Inhibits Triglycerides Accumulation during Murine Adipocytes' and Human Adipose-Derived Mesenchymal Stem Cells Differentiation
Abstract
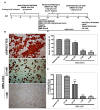
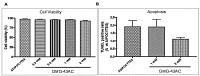
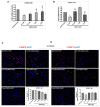
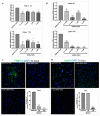
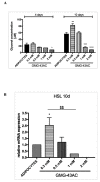
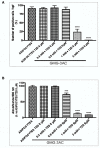
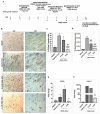
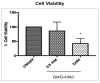
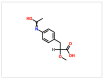
Understanding the molecular basis of adipogenesis is vital to identify new therapeutic targets to improve anti-obesity drugs. The adipogenic process could be a new target in the management of this disease. Our aim was to evaluate the effect of GMG-43AC, a selective peroxisome proliferator-activated receptor γ (PPARγ) modulator, during adipose differentiation of murine pre-adipocytes and human Adipose Derived Stem Cells (hADSCs). We differentiated 3T3-L1 cells and primary hADSCs in the presence of various doses of GMG-43AC and evaluated the differentiation efficiency measuring lipid accumulation, the expression of specific differentiation markers and the quantification of accumulated triglycerides. The treatment with GMG-43AC is not toxic as shown by cell viability assessments after the treatments. Our findings demonstrate the inhibition of lipid accumulation and the significant decrease in the expression of adipocyte-specific genes, such as PPARγ, FABP-4, and leptin. This effect was long lasting, as the removal of GMG-43AC from culture medium did not allow the restoration of adipogenic process. The above actions were confirmed in hADSCs exposed to adipogenic stimuli. Together, these results indicate that GMG-43AC efficiently inhibits adipocytes differentiation in murine and human cells, suggesting its possible function in the reversal of adipogenesis and modulation of lipolysis.
Keywords: PPAR γ modulator; adipocytes; adipogenesis; differentiation; lipogenesis.
Conflict of interest statement
G.G. serves as vice president of Giuliani SpA. B.M. is an employee of this company. No other relationships/conditions/circumstances present a potential conflict of interest.
Figures




References
-
- Sarbatly R., Krishnaiah D., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233.
-
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

